Antibody for resisting staphylococcus aureus toxin and applications thereof
A staphylococcal, golden-yellow technology, applied in the direction of antibodies, antibacterial drugs, antibacterial immunoglobulin, etc., can solve tissue necrosis and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
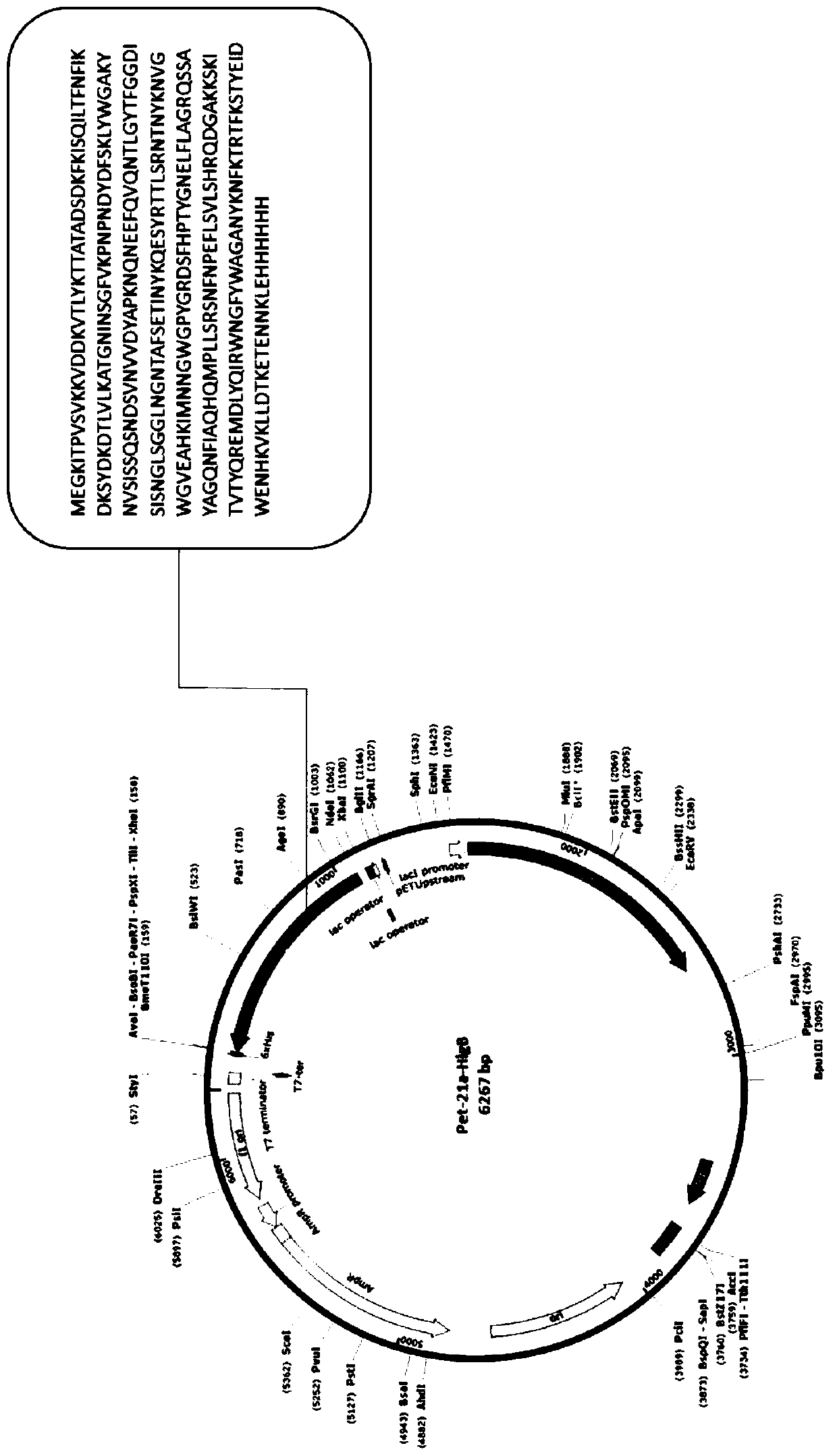
[0049] Example 1: Recombinant expression of Candida albicans HlgB protein fused to His tag
[0050] Taking the amino acids of HlgB and HlgA proteins of Staphylococcus aureus as the target sequences, the corresponding base sequences were artificially synthesized, and cloned into the Pet-21a plasmid containing the His tag by using restriction sites NdeI and XhoI. The recombinant plasmid was transformed into competent cells BL21(DE3)pLysS, and a single colony was picked and inoculated into LB liquid medium containing 100 μg / ml ampicillin the next day, and cultured overnight at 37°C with shaking. The overnight culture solution was inoculated into LB liquid medium containing 100 μg / ml ampicillin at a ratio of 1:100, and cultured with shaking at 200 rpm at 37°C until OD 600 About 0.6-0.8, add IPTG to the bacterial solution to a final concentration of 0.5mM, and induce at 37°C for 4.5h. Take the induced bacterial liquid, centrifuge at 8,000 rpm for 3 minutes to collect the bacterial...
Embodiment 2
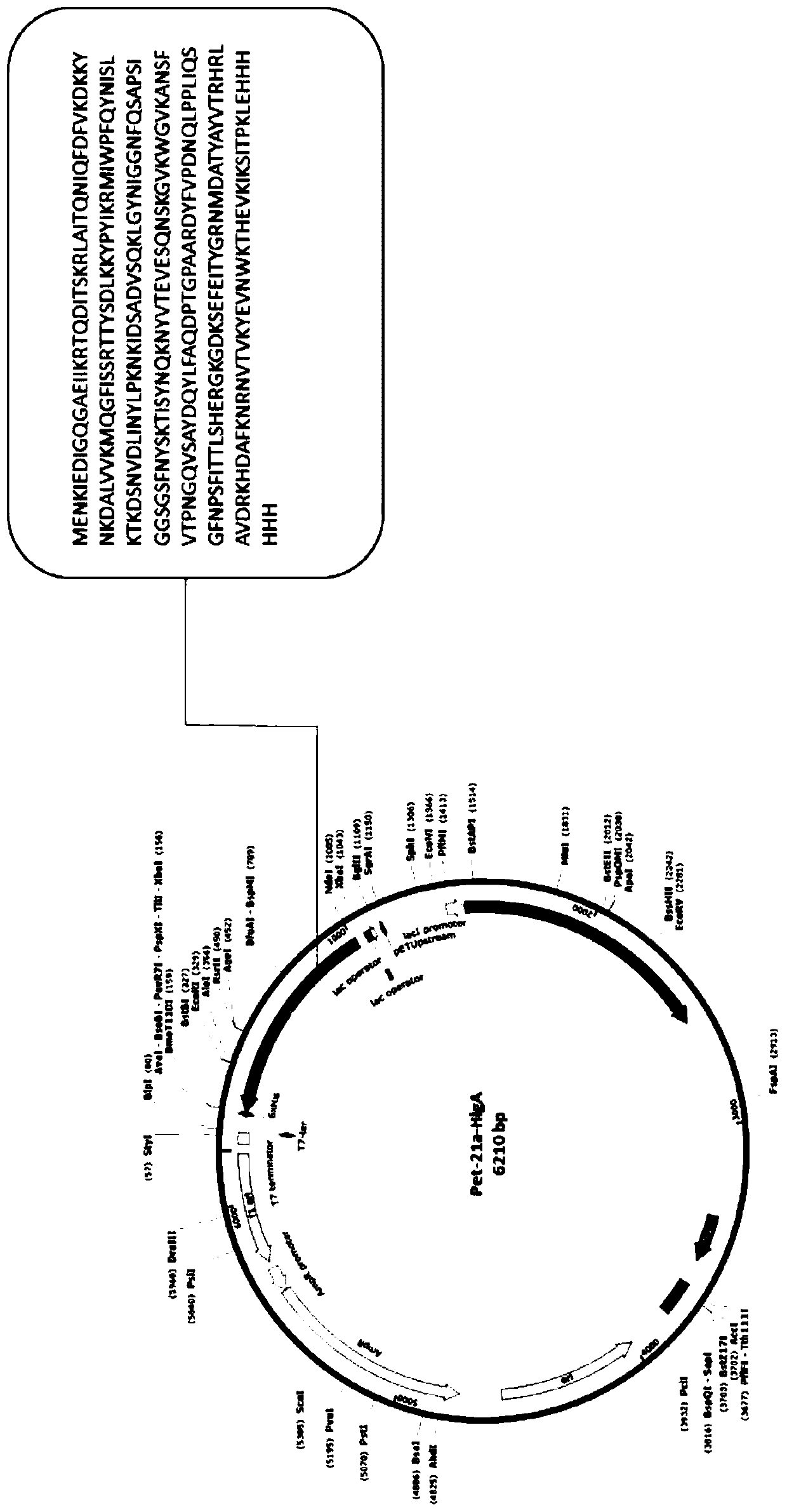
[0055] Example 2: Recombinant expression of HlgA protein fused to His tag
[0056] Taking the amino acid of Staphylococcus aureus HlgA protein as the target sequence, the corresponding base sequence was artificially synthesized, and cloned into the Pet-21a plasmid containing His tag by using restriction sites NdeI and XhoI. The recombinant plasmid was transformed into competent cells BL21(DE3)pLysS, and a single colony was picked and inoculated into LB liquid medium containing 100 μg / ml ampicillin the next day, and cultured overnight at 37°C with shaking. The overnight culture solution was inoculated into LB liquid medium containing 100 μg / ml ampicillin at a ratio of 1:100, and cultured with shaking at 200 rpm at 37°C until OD 600 About 0.6-0.8, add IPTG to the bacterial solution to a final concentration of 0.5mM, and induce at 37°C for 4.5h. Take the induced bacterial liquid, centrifuge at 8,000 rpm for 3 minutes to collect the bacterial cells, and store at -80°C.
[0057] ...
Embodiment 3
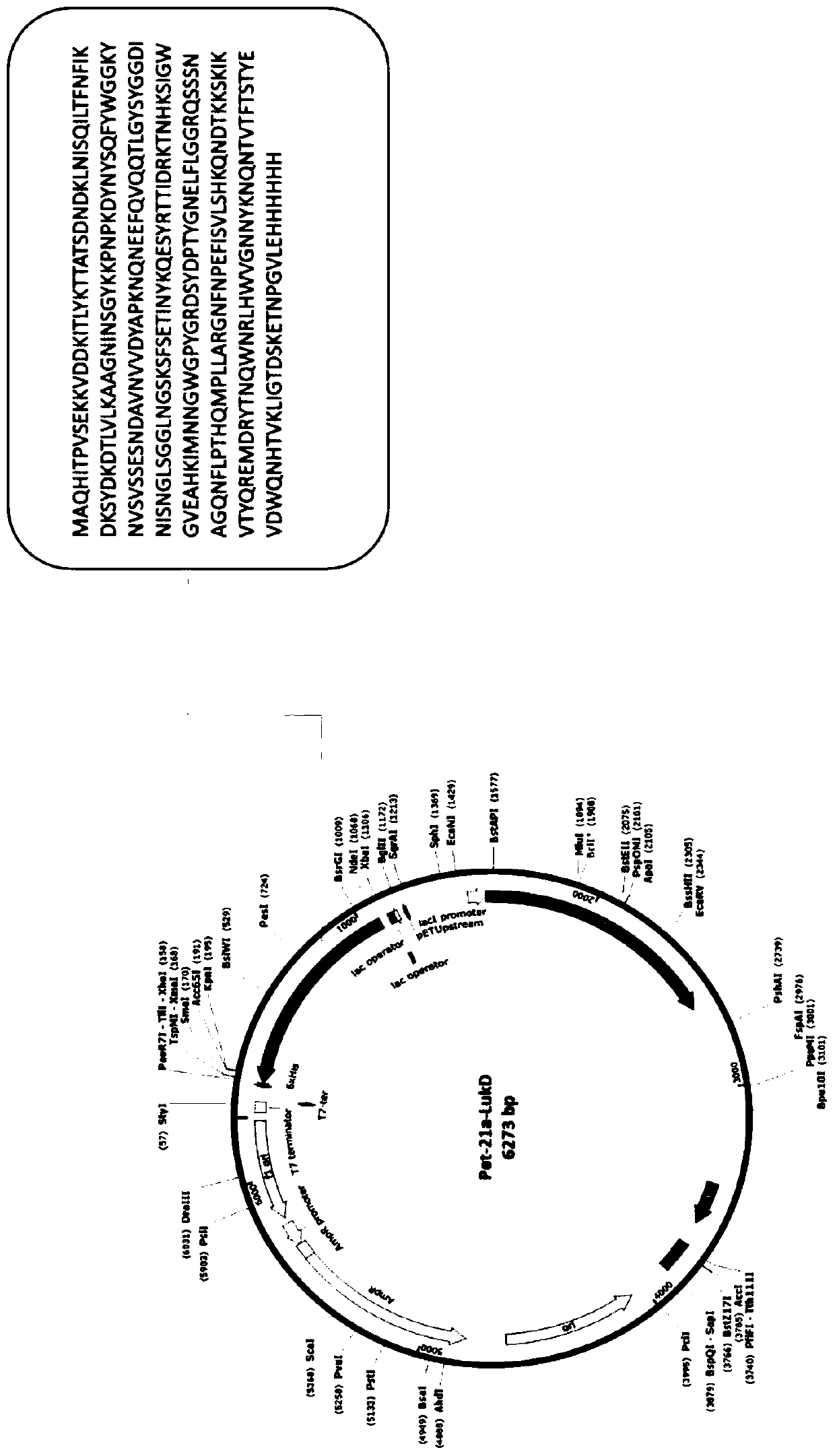
[0061] Example 3: Recombinant expression of HlgC protein fused to His tag
[0062] Taking the amino acid of Staphylococcus aureus HlgC protein as the target sequence, the corresponding base sequence was artificially synthesized, and cloned into the Pet-21a plasmid containing His tag by using restriction sites NdeI and XhoI. The recombinant plasmid was transformed into competent cells BL21(DE3)pLysS, and a single colony was picked and inoculated into LB liquid medium containing 100 μg / ml ampicillin the next day, and cultured overnight at 37°C with shaking. The overnight culture solution was inoculated into LB liquid medium containing 100 μg / ml ampicillin at a ratio of 1:100, and cultured with shaking at 200 rpm at 37°C until OD 600 About 0.6-0.8, add IPTG to the bacterial solution to a final concentration of 0.5mM, and induce at 37°C for 4.5h. Take the induced bacterial liquid, centrifuge at 8,000 rpm for 3 minutes to collect the bacterial cells, and store at -80°C.
[0063] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com