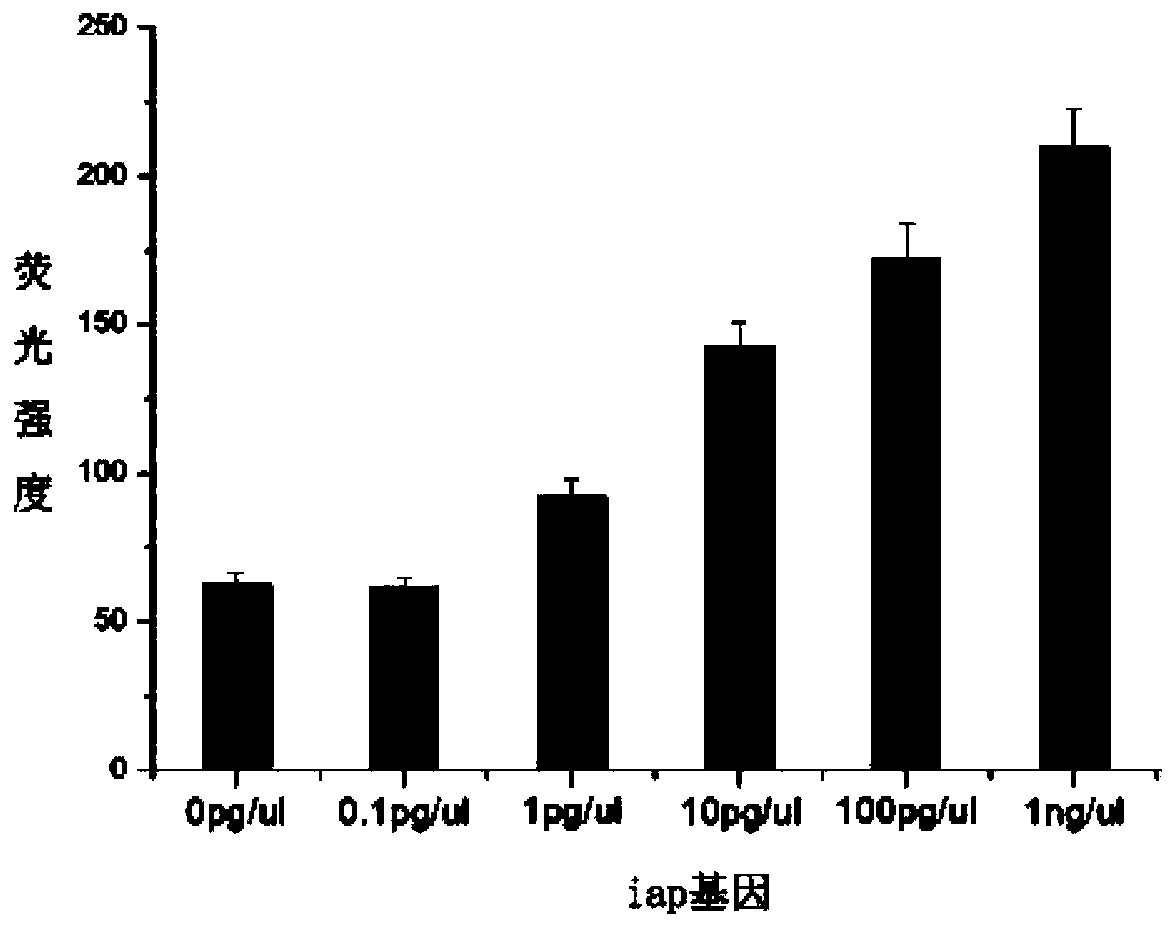
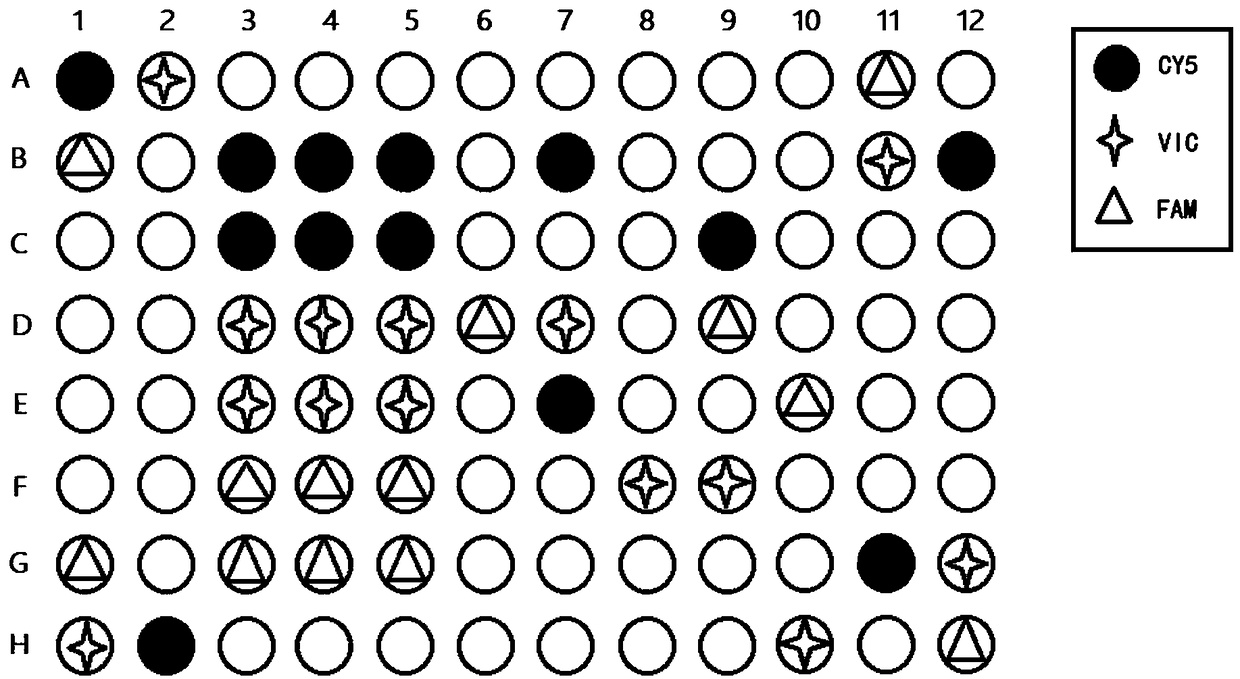
Patents
Literature
34results about How to "Highly pathogenic" patented technology
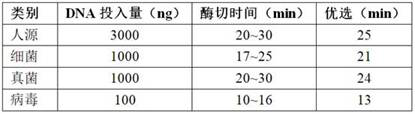
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
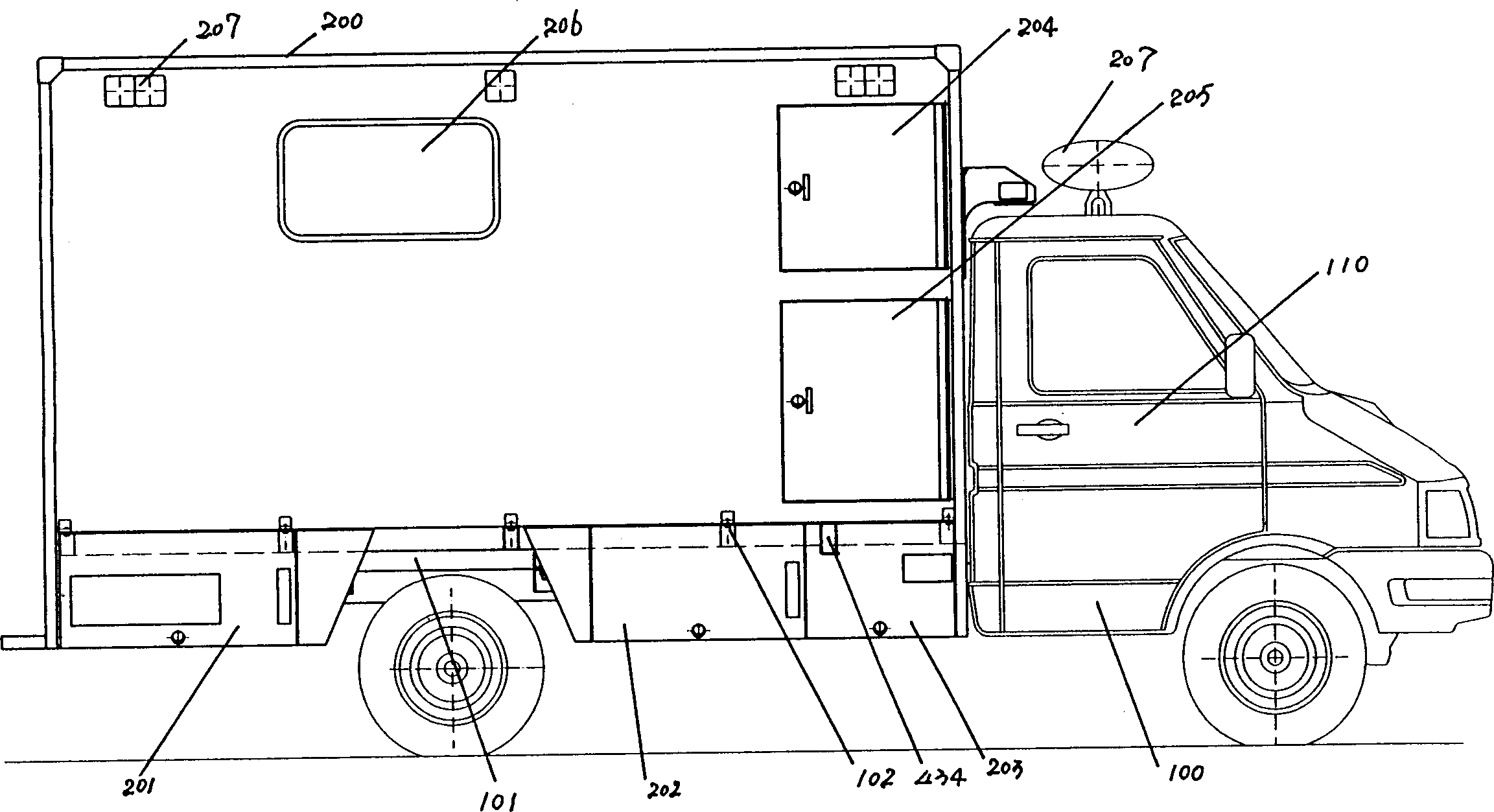
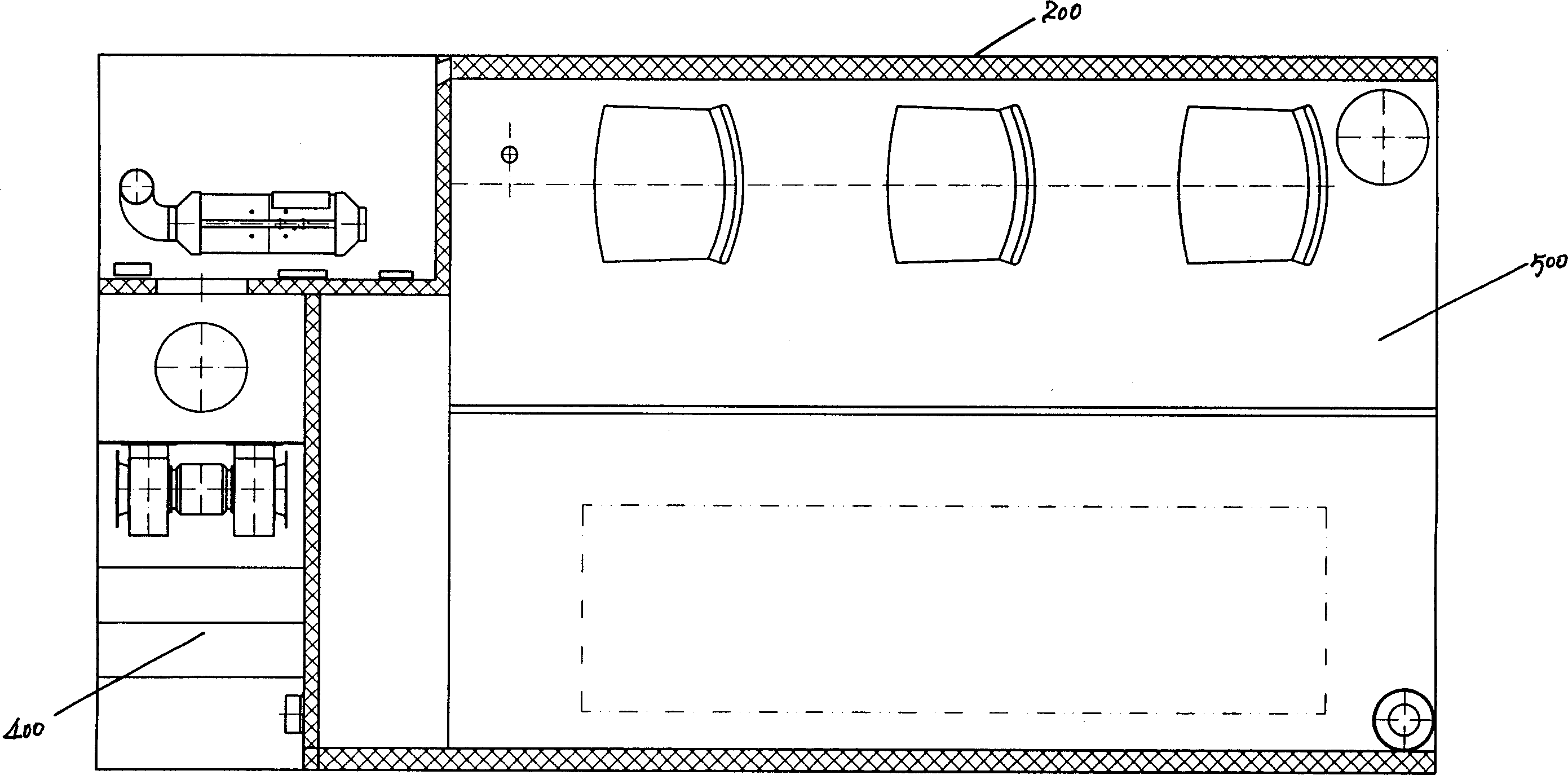
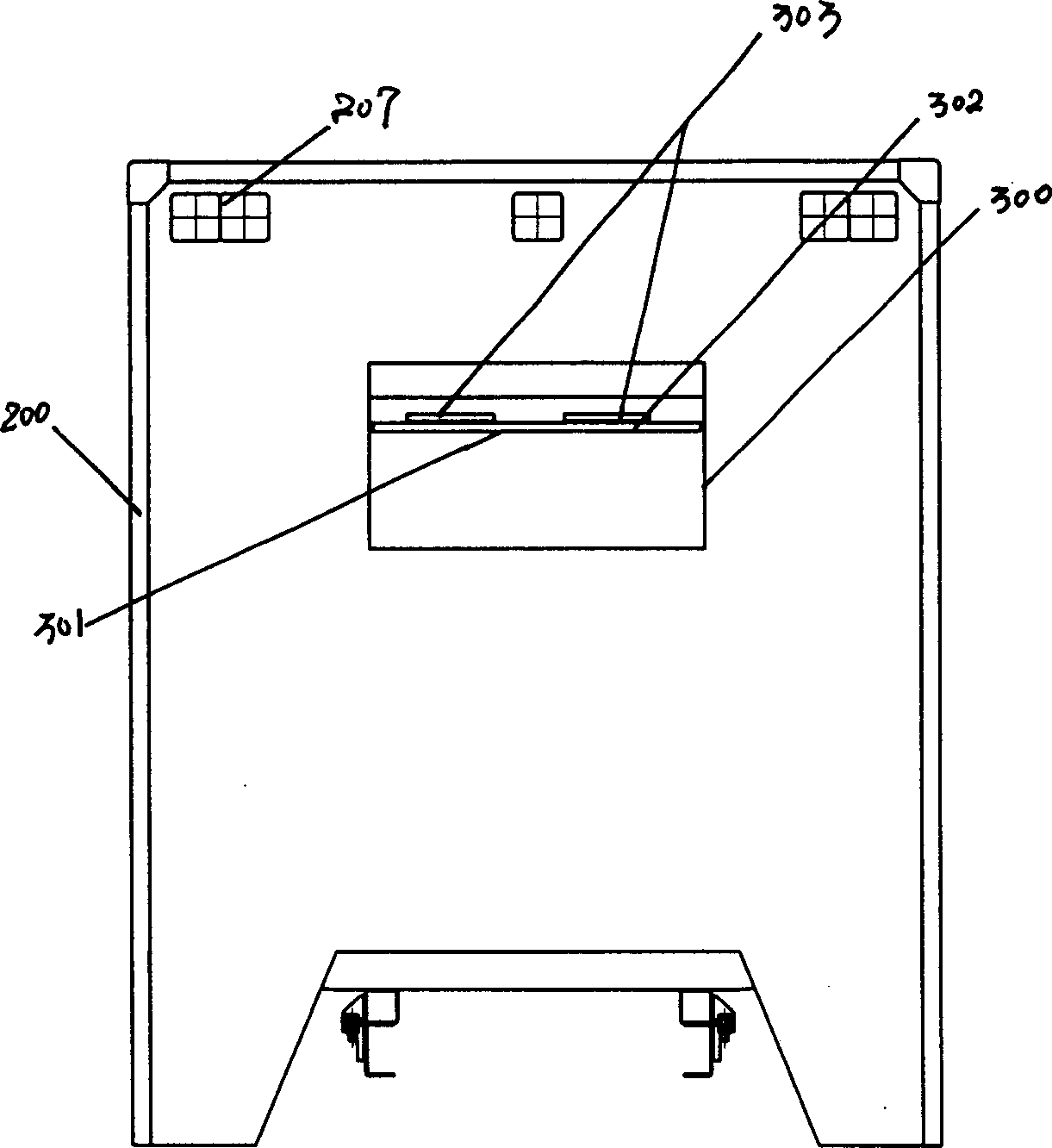
Negative pressure ambulance capable of preventing cross infection of doctor and patient
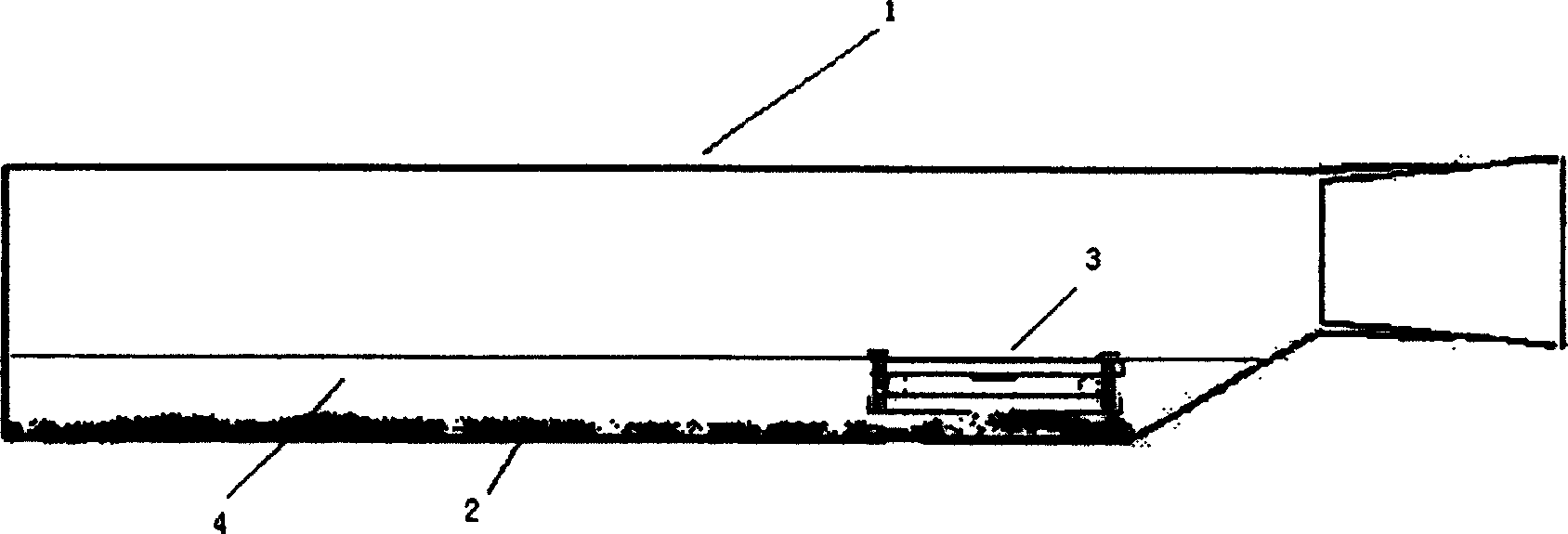
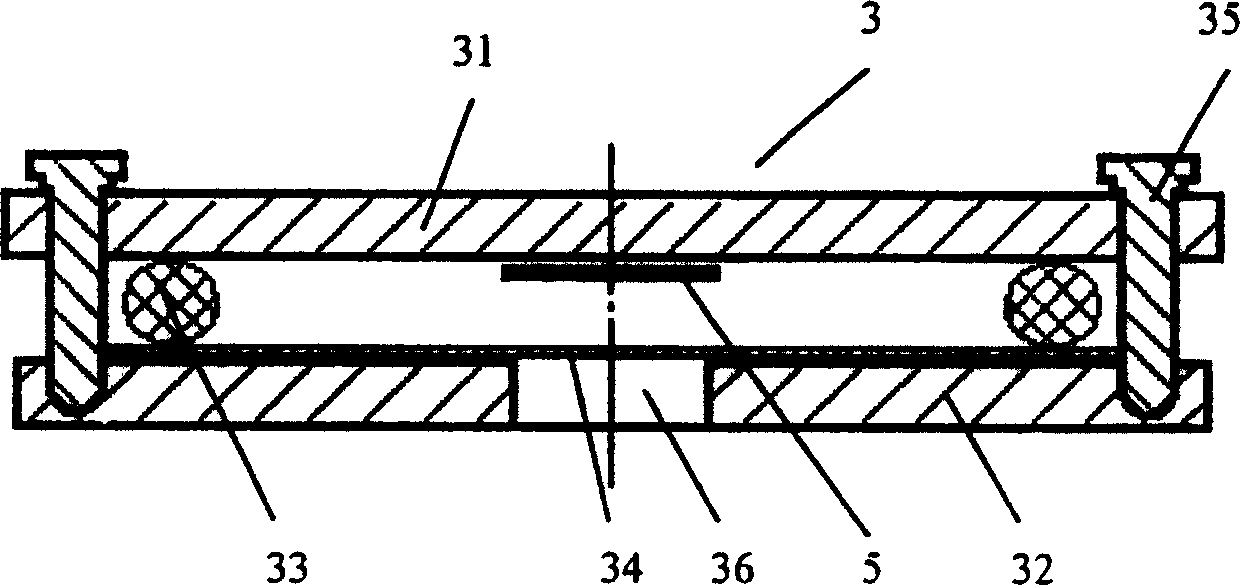
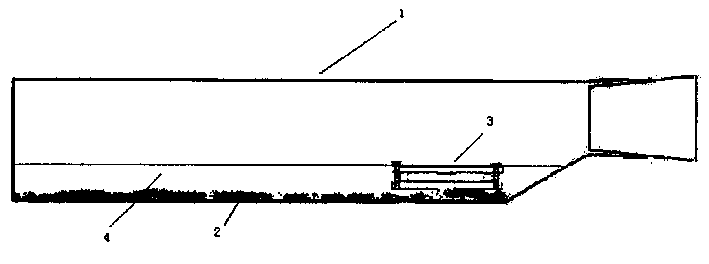
The invention discloses a negative pressure ambulance used to prevent nosocomial infection, containing car body, hermetic board cabin connected with car body chassis, control device; the hermetic board cabin is consisted of nose functional cabin and medical afterhold; the nose functional cabin is the flow direction of air controlling cell, the medical afterhold contains cabin air inlet chamber, cabin exhaust chamber, also a medical equipment in cabin; the medical afterhold sets closable port; the cabin air inlet chamber of medical afterhold and air mixing chamber of nose functional cabin can be connected, the cabin exhaust chamber of medical afterhold and the air filtration disinfection chamber can be connected. The air flow of hermetic board cabin has three kinds of state, the air in cabin can all realize directional airflow and pressure gradient. This car can be used as normal ambulance, high pathogenic infectious patient ambulance, various diseases checking car, mobile p3 lab, carrier vehicle of biological bacteria danger foe things; it can be asepsis operation car after switching, high infectivity, strong virulence weapon battlefield command, arm carrying car.
Owner:北京华瑞核安科技股份有限公司
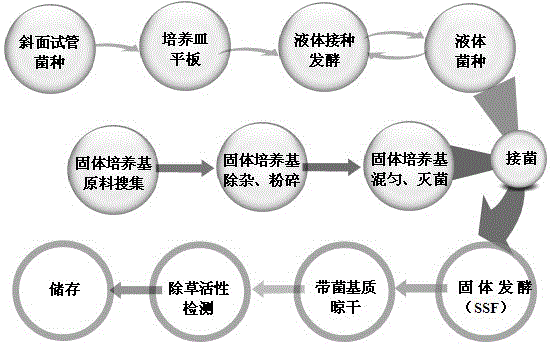
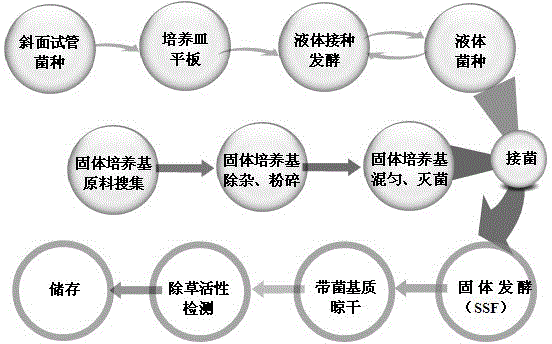
Sclerotium rolfsii solid fermentation culture medium and application thereof
The invention discloses a sclerotium rolfsii solid fermentation culture medium and an application thereof. The solid fermentation culture medium comprises a solid substrate and water, wherein the solid substrate comprises wheat bran and agricultural wastes, the wheat bran accounts for 20%-60% of the total weight of the solid substrate, and the weight ratio of the water to the solid substrate is (20-45):100; and the agricultural wastes select rice husk, sweet sorghum straw, an organic fertilizer, a wheat bran substrate, vinegar residue, wine lees, garden dead twigs or sawdust. The sclerotium rolfsii solid fermentation culture medium prepared by the preparation method disclosed by the invention is low in cost, the preparation process is simple, and a herbicide prepared by adopting the solid fermentation culture medium can be preserved at room temperature.
Owner:NANJING AGRICULTURAL UNIVERSITY
Method for preparing high efficiency biological weed control bacterial agent and its usage
A process for preparing the efficient bacterial herbicide includes sampling the soil near the root of weeds such as crabgrass herb, caper euphorbia, cassia, etc. or their stem or leaves, separating with NPC culture medium, and the chlorella pyrenoidosa and glutamine synthetase depressants, and screening the bacterial strains with high herbiciding activity and broad spectrum. Its advantage is highherbiciding effect.
Owner:ZHEJIANG UNIV
Multi-gene detection method of Listeria monocytogenes based on quantum dot/graphene oxide nanometer platform
InactiveCN103233078AHigh sensitivityThe detection process is simpleMicrobiological testing/measurementFluorescence/phosphorescenceFluorescencePolymerase chain reaction
The invention discloses a multi-gene detection method of Listeria monocytogenes based on a quantum dot / graphene oxide nanometer platform and belongs to the technical field of gene nanometer detection. The multi-gene detection method of Listeria monocytogenes comprises the following steps of: selecting two or more than two genes of the Listeria monocytogenes as target points of genetic detection and designing two pairs or more than two pairs of genetic PCR (Polymerase Chain Reaction) amplimers according to the analysis result of a gene conserved area; amplifying two or more than two genetic special sequences at the same time according to the principle of LATE-PCR to obtain a corresponding single stranded amplification product; then, hybridizing the single stranded amplification product with a quantum dot fluorescence probe; and finally, quenching and removing the non-hybridized quantum dot probe by using graphene oxide. When the target gene does not exist, all quantum dot fluorescence probes are quenched. On the contrary, when the target gene exists, corresponding fluorescent signals are obtained. The detection method has high detection reliability to Listeria monocytogenes.
Owner:SOUTH CHINA NORMAL UNIVERSITY
Negative pressure ambulance capable of preventing cross infection of doctor and patient
The invention discloses a negative pressure ambulance used to prevent nosocomial infection, containing car body, hermetic board cabin connected with car body chassis, control device; the hermetic board cabin is consisted of nose functional cabin and medical afterhold; the nose functional cabin is the flow direction of air controlling cell, the medical afterhold contains cabin air inlet chamber, cabin exhaust chamber, also a medical equipment in cabin; the medical afterhold sets closable port; the cabin air inlet chamber of medical afterhold and air mixing chamber of nose functional cabin can be connected, the cabin exhaust chamber of medical afterhold and the air filtration disinfection chamber can be connected. The air flow of hermetic board cabin has three kinds of state, the air in cabin can all realize directional airflow and pressure gradient. This car can be used as normal ambulance, high pathogenic infectious patient ambulance, various diseases checking car, mobile p3 lab, carrier vehicle of biological bacteria danger foe things; it can be asepsis operation car after switching, high infectivity, strong virulence weapon battlefield command, arm carrying car.
Owner:北京华瑞核安科技股份有限公司
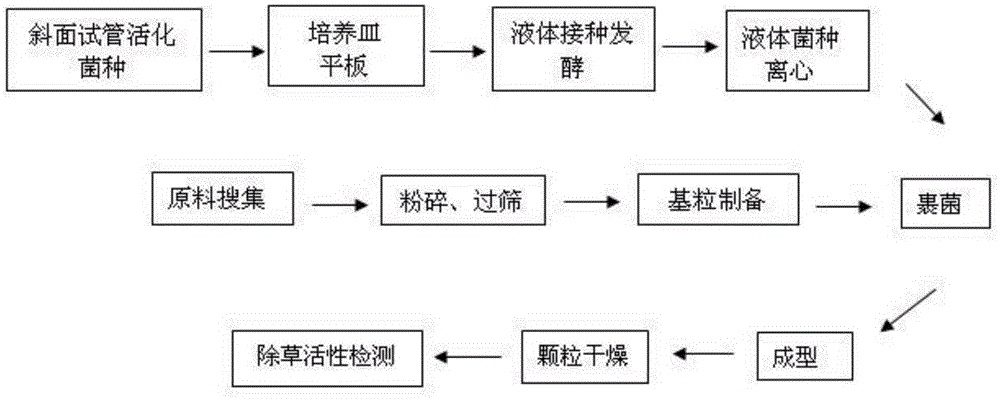
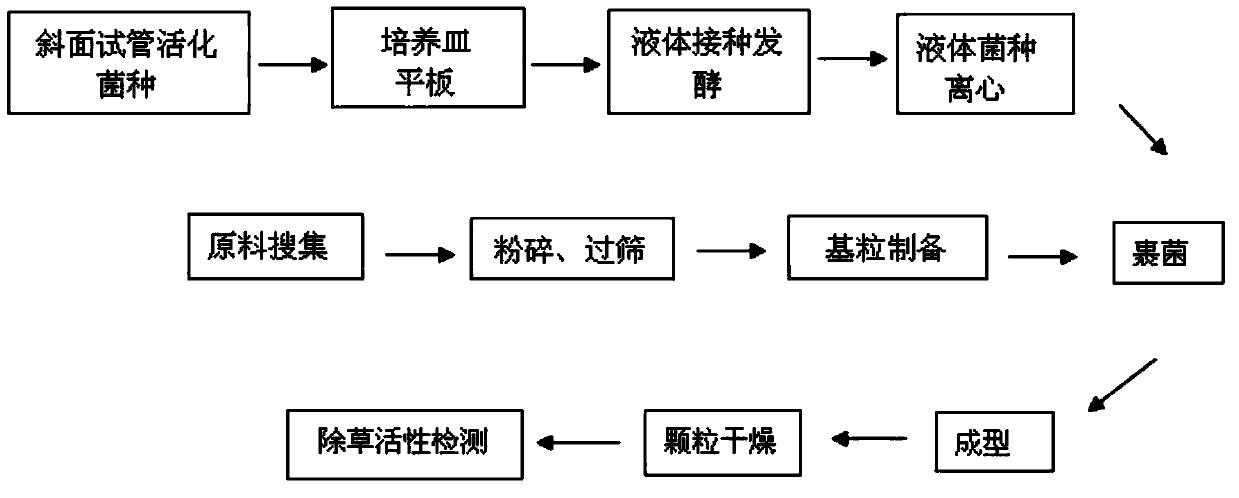
Biological weeding organic fertilizer, and preparation method and application thereof
ActiveCN105330395AImprove stress resistanceImprove qualityPlant protectionFertilizer mixturesAdhesiveSolid particle
The invention discloses a biological weeding organic fertilizer. The biological weeding organic fertilizer is a solid granule prepared through wrapping a base granule with Sclerotium rolfsii Sacc. SC64 mycelia; a mass ratio of the Sclerotium rolfsii Sacc. SC64 mycelia to the base granule s 1.7-2.0:1; and the base granule is prepared from, by mass, 5-45% of an organic fertilizer, 1.3-28.6% of an adhesive, 10-15% of water, and the balance of a solid matrix. The invention also discloses a preparation method of the biological weeding organic fertilizer, and an application of the biological weeding organic fertilizer in control of dicotyledon weeds. The biological weeding organic fertilizer simultaneously has the advantages of a traditional organic fertilizer and a biological herbicide, so garden wastes and crop straws are fully used to realize changing of wastes into valuables and reduce environment pollution caused by straw burning, and the use of chemical fertilizers and pesticides is reduced to enhance the stress resistance of crops and improve the quality of the crops.
Owner:NANJING AGRICULTURAL UNIVERSITY
Fusarium oxysporum strain and application thereof
ActiveCN110616157AHighly pathogenicNo tissue specificityFungiMicrobiological testing/measurementDiseaseHighly pathogenic
The present invention discloses a fusarium oxysporum strain and belongs to the technical field of pathogenic microorganism isolation. The fusarium oxysporum is fusium oxysporum Hs-f (Fusarium sp.), isdeposited on August 29, 2019 in China General Microbiological Culture Collection Center and has a deposit number of CGMCC NO:18130, the address is No.3, No.1 courtyard, Beichen West Road, Chaoyang District, Beijing, and a unit asking for deposition is Peanut Institute of Shandong province. The peanut rot pathogen fungus is isolated and purified from peanut rot disease seed kernels. Inoculation experiments prove that the fusarium oxysporum strain has high pathogenicity, is also free of tissue specificity, and has pathogenicity to peanut roots, stems, leaves and seed kernels.
Owner:SHANDONG PEANUT RES INST +1
Avian infectious bronchitis virus natural mutant
InactiveCN105624122ABreakthrough immune protectionHighly pathogenicSsRNA viruses positive-senseMicroorganism based processesLaboratory cultureMutation
The invention relates to the field of virology and aims to provide an avian infectious bronchitis virus natural mutant, kept in China General Microbiological Culture Collection Center, named infectious bronchitis virus and collected under CGMCC No. 11491. A genome of this mutant is 27, 541 lbp in length, a complete genome sequence of the mutant is as shown in SEQ ID NO: 1, with structural characteristics of a typical IBV genome coding structure. This mutant having special genes inserted in and provided by the invention can break the immunity protection of vaccines to breed in an organism, this mutant can proliferate in susceptible chickens without causing significant pathogenicity to sensitive chickens, and thus this mutant can serve as base material for the further study on mutation mechanism of an attenuated vaccine or IBV virus strain.
Owner:ZHEJIANG UNIV
Generic assay for detection of influenza viruses
InactiveUS20120201851A1Highly pathogenicEasy to modifySsRNA viruses negative-senseMicrobiological testing/measurementAssayVaccine Production
The invention relates to generic methods for the detection and quantification of influenza viruses. These may uses a reverse transcription (RT-PCR) real time (q-PCR) assay which amplifies a conserved region within influenza A or B strains. The assays allow the quantification of influenza virus RNA molecules or whole virus particles, irrespective of the particular virus strain (e.g. human, avian, swine flu). The methods are particularly applicable as diagnostic assays or in the monitoring of vaccine production processes.
Owner:NOVARTIS AG
Blocking agent capable of inhibiting porcine reproductive and respiratory syndrome virus infection
ActiveCN109701021AReduced inhibitory activityHighly pathogenicOrganic active ingredientsPeptide/protein ingredientsProtein targetNotch signalling pathway
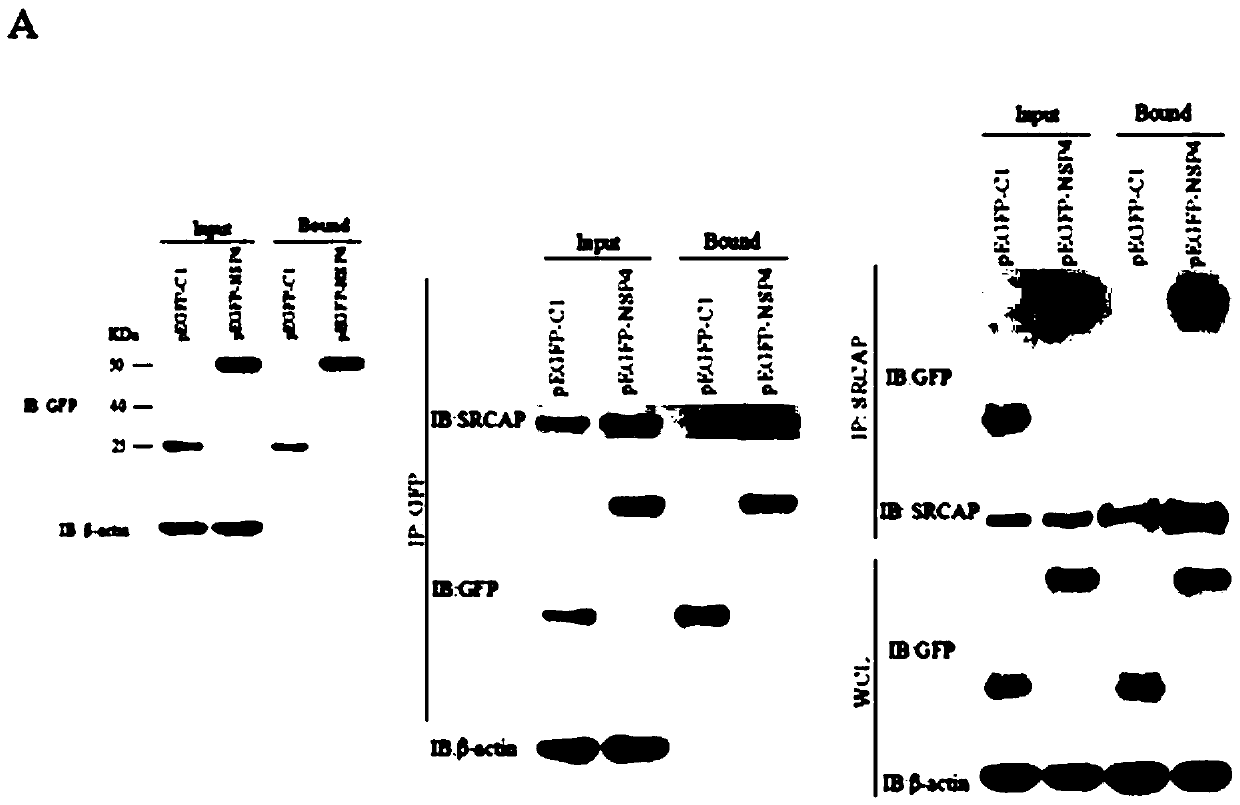
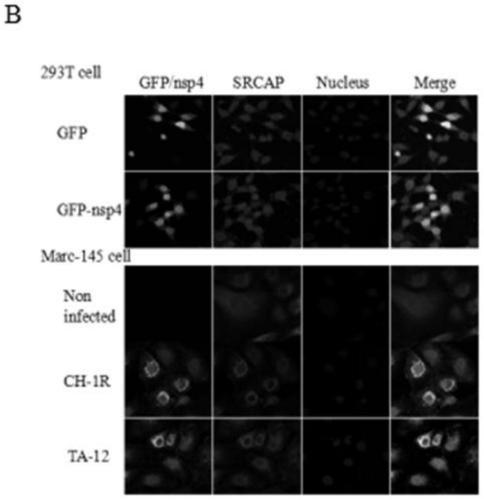
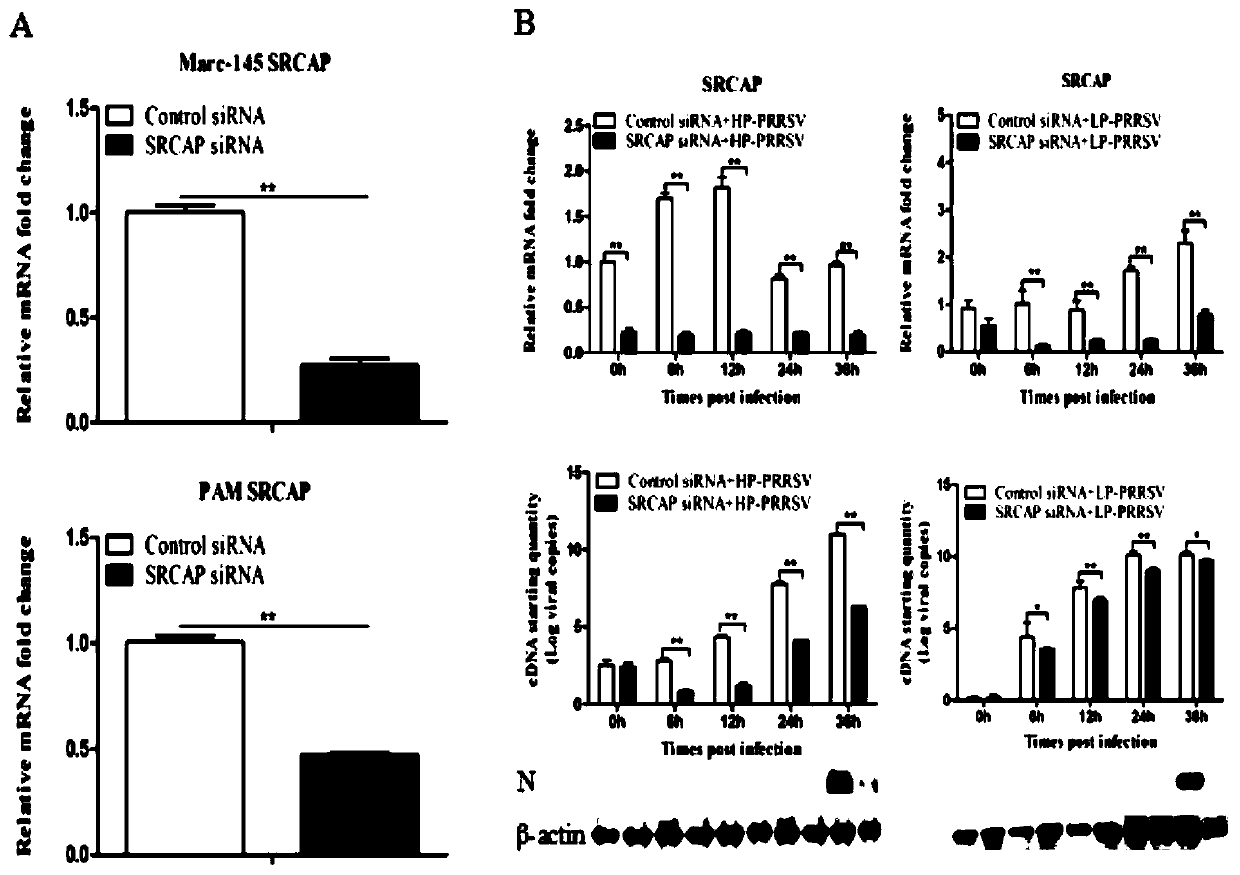
The invention discloses a blocking agent capable of inhibiting porcine reproductive and respiratory syndrome virus (PRRSV) infection. The invention discovers a new PRRSV cell target protein, namely Snf2-related CBP activator protein (SRCAP), and finds out inhibitory targets of the receptor. PRRSV infection can be significantly reduced by interfering SRCAP genes or using a Notch signaling pathway inhibitor, namely a gamma-secretase inhibitor, in which the SRCAP protein plays a key role at the inhibitory targets; and thus, the inhibitory targets and the gamma-secretase inhibitor can be developedinto drugs used for preventing and treating PRRSV infection, thereby providing brand new ideas for PRRSV research as well as prevention and treatment. Therefore, scope of research is expanded; and thus, the blocking agent is of great significance to practical production.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Porcine reproductive and respiratory syndrome virus recombinant vaccine strain PRRSV-SP as well as preparation method and application thereof
ActiveCN110904055ANo apparent pathogenicityHighly pathogenicSsRNA viruses positive-senseViral antigen ingredientsHeterologousHighly pathogenic
The invention provides a porcine reproductive and respiratory syndrome virus recombinant vaccine strain PRRSV-SP as well as a preparation method and application thereof. The T3448A site of a vaccine strain SP is mutated to obtain the recombinant vaccine strain PRRSV-SP. According to the method, genes ORF5 and ORF6 of main structural proteins GP5 and M of a highly pathogenic porcine reproductive and respiratory syndrome virus Hubei isolate strain HUB2 are put into corresponding coding regions of the recombinant vaccine strain PRRSV-SP for expression, so that a chimeric toxic strain vSP-Hub2 isobtained. The recombinant vaccine strain PRRSV-SP and the chimeric toxic strain vSP-Hub2 provided by the invention have no obvious pathogenicity, can be used as the basic material for further studyingthe mutation mechanism of an attenuated vaccine and a PRRSV toxic strain, can construct a novel attenuated vaccine strain through an infectious cloning system, and can be used for preparing a novel,efficient and broad-spectrum vaccine against PRRSV homologous and heterologous toxic strains.
Owner:SOUTH CHINA AGRI UNIV
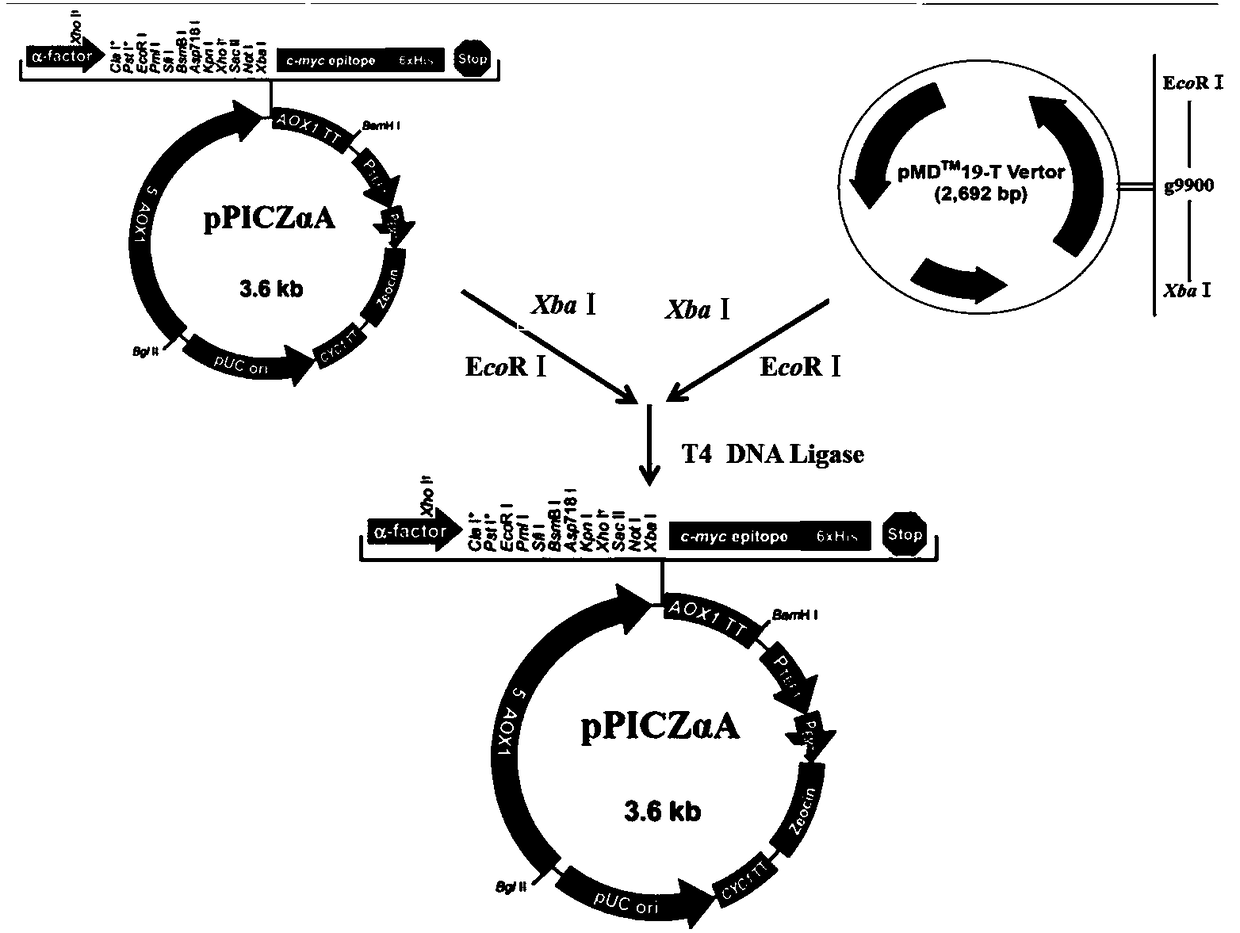
Nimbya alternantherae effector Na2-g9900, and protein and application thereof
The invention discloses a Nimbya alternantherae effector Na2-g9900, and a protein and an application thereof. The nucleotide full-length sequence of the effector gene is represented by SEQ ID NO.1, the open reading frame sequence is represented by SEQ ID NO.2, and the amino acid sequence of the effector Na2-g9900 protein is represented by SEQ ID NO.3. The effector Na2-g9900 found IN the inventionhas significant pathogenicity to Alternanthera Philoxeroides, can be used for preparing preparation products for controlling Alternanthera Philoxeroides, can also be used for constructing biocontrol bacteria, and has an excellent application prospect in the treatment of Alternanthera philoxeroides.
Owner:ZHONGKAI UNIV OF AGRI & ENG
Bio-organic fertilizer as well as production method and application thereof
Owner:JIANGSU DONGBAO FERTILIZER CO LTD
Influenza virus a subtype somatotype detecting method
ActiveCN108977585AQuick checkSensitive detectionMicrobiological testing/measurementForward primerRNA extraction
The invention discloses a influenza virus a subtype somatotype detecting method. The method comprises the following steps that 1, a primer is designed and synthesized; 2, carboxyl magnetic beads connected with the forward primer are prepared; 3, the influenza virus a RNA is extracted; 4, RT-PCR (reverse transcriptase-polymerase chain reaction) is conducted; 5, magnetic adsorption and washing are conducted; and 6, detecting is conducted. The influenza virus a subtype somatotype detecting method can conduct classification on the subtypes of the influenza virus a rapidly, sensitively and specifically and at the high flux, and has the very good application value.
Owner:宁波怡和医药科技有限公司
Alternaria pseudoalpacia effector na2-g9900 and its protein and application
The invention discloses an Alternaria pseudoalpacia effector Na2‑g9900 And its protein and application. The full-length nucleotide sequence of the effector gene is shown in SEQ ID NO.1, the open reading frame sequence is shown in SEQ ID NO.2, and the amino acid sequence of the effector Na2-g9900 protein is shown in SEQ ID NO.3 . The effector Na2-g9900 found in the present invention has significant pathogenicity to A. japonicus, can be used to prepare preparations for preventing and treating A. japonicus, and can also be used to construct biocontrol bacteria. It has a good effect on the control of A. japonicus application prospects.
Owner:ZHONGKAI UNIV OF AGRI & ENG
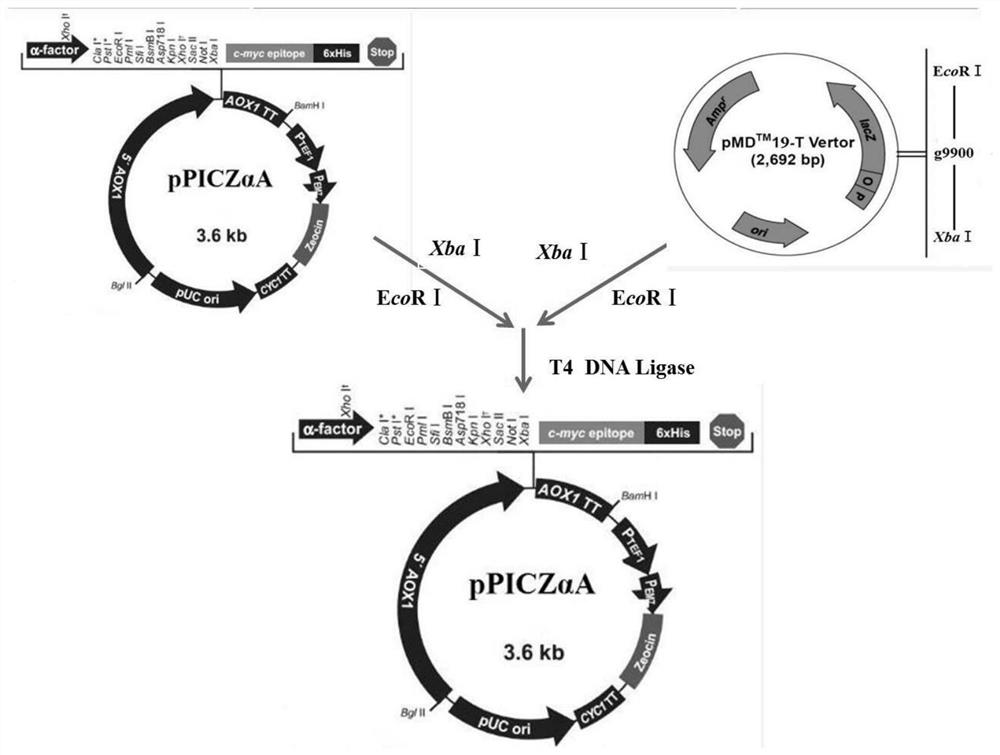
Transgenic sugar beet event gm rz13
InactiveUS20120144516A1Stably integratedReliable controlSugar derivativesOrganic compound preparationGenotypeInsertion site
A novel transgenic sugar beet event designated GM RZ13 is disclosed. The invention relates to nucleic acids that are unique to event GM RZ13. The invention also relates to assays for detecting the presence of the GM RZ13 event based on DNA sequences of the recombinant constructs inserted into the sugar beet genome that resulted in the GM RZ13 event and of genomic sequences flanking the insertion site. The invention further relates to sugar beet plants comprising the genotype of GM RZ13 and to methods for producing a sugar beet plant by crossing a sugar beet plant comprising the GM RZ13 genotype with itself or another sugar beet variety. Seeds of sugar beet plants comprising the GM RZ13 genotype are also objects of the present invention.
Owner:SYNGENTA PARTICIPATIONS AG
A kind of biological weeding organic fertilizer and its preparation method and application
The invention discloses a biological weeding organic fertilizer. The biological weeding organic fertilizer is a solid granule prepared through wrapping a base granule with Sclerotium rolfsii Sacc. SC64 mycelia; a mass ratio of the Sclerotium rolfsii Sacc. SC64 mycelia to the base granule s 1.7-2.0:1; and the base granule is prepared from, by mass, 5-45% of an organic fertilizer, 1.3-28.6% of an adhesive, 10-15% of water, and the balance of a solid matrix. The invention also discloses a preparation method of the biological weeding organic fertilizer, and an application of the biological weeding organic fertilizer in control of dicotyledon weeds. The biological weeding organic fertilizer simultaneously has the advantages of a traditional organic fertilizer and a biological herbicide, so garden wastes and crop straws are fully used to realize changing of wastes into valuables and reduce environment pollution caused by straw burning, and the use of chemical fertilizers and pesticides is reduced to enhance the stress resistance of crops and improve the quality of the crops.
Owner:NANJING AGRICULTURAL UNIVERSITY
A virulent strain of Mycoplasma bovis and its application
ActiveCN110317749BHigh re-isolation rateStrong titerBacteriaMicrobiological testing/measurementEngineeringVirus strain
The invention provides a virulent strain of mycoplasma bovis and an application thereof, belonging to the technical field of microbes. The virulent Mycoplasma bovis strain of the present invention is classified as Mycoplasma bovis (Mycoplasma bovis) 16M strain, and the preservation number of the China Center for Type Culture Collection (CCTCC) is M2019235. This strain can induce a variety of typical clinical symptoms of Mycoplasma bovis infection, including asthma and obvious pulmonary consolidation, and has a strong invasive force on the lungs of cattle, with high titer of viable bacteria, strong virulence, and high pathogenicity , the time of sick beef steak bacteria is long, the onset is fast, and the isolation rate of the lung virus strain after the challenge is high, which can be used to establish the Mycoplasma bovis pathogenesis model with typical symptoms, which meets the clinical characteristics of the cattle farm. Therefore, the strong Mycoplasma bovis strain of the present invention has Good practical value.
Owner:DAIRY CATTLE RES CENT SHANDONG ACADEMY OF AGRI SCI
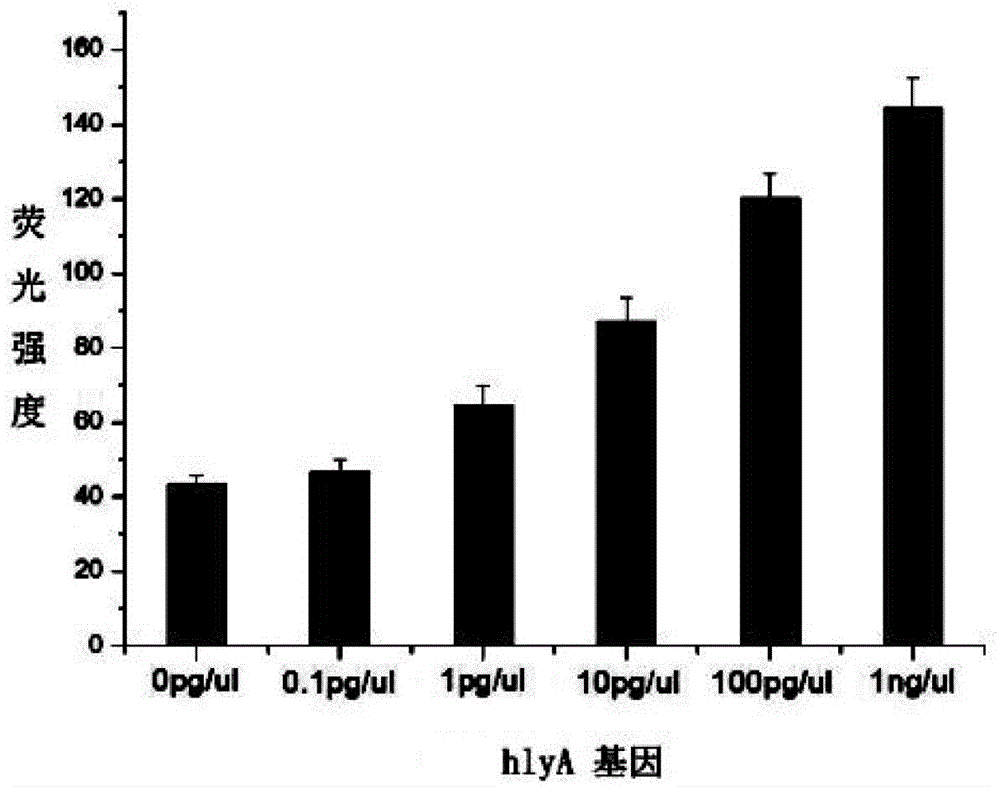
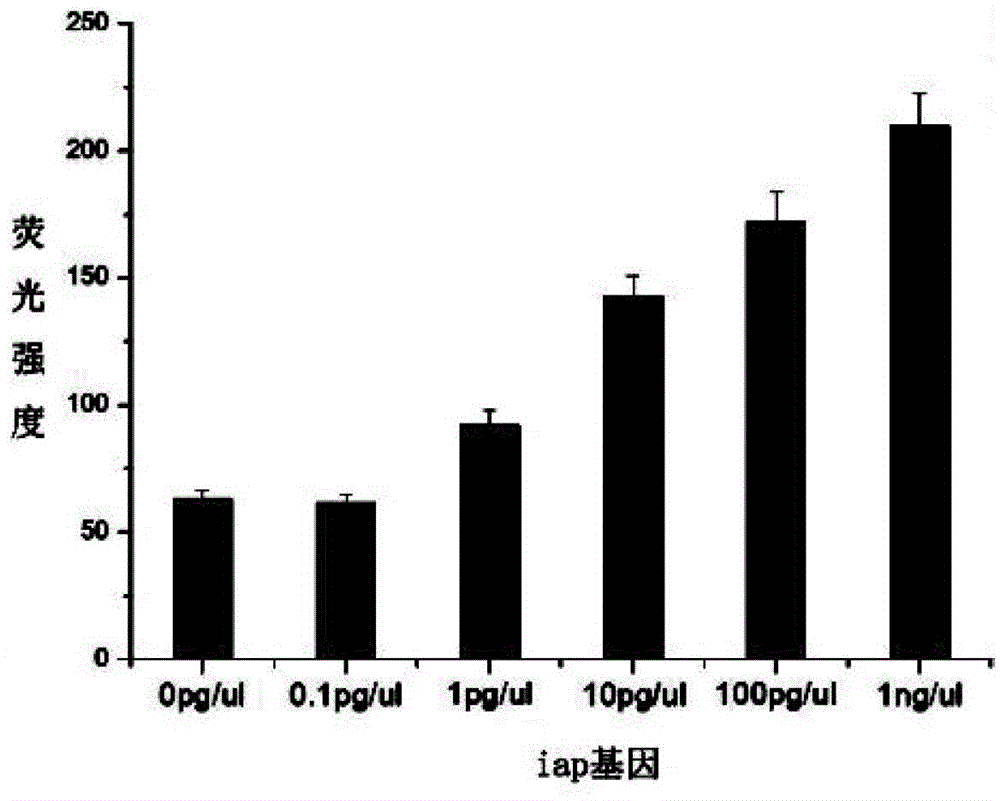
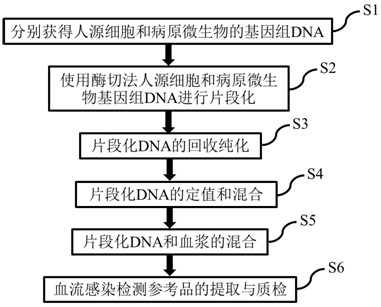
Reference product for detection of pathogenic microorganisms in bloodstream infection and preparation method thereof
ActiveCN113249441BReliable sourceHighly pathogenicMicrobiological testing/measurementPathogenic microorganismReference product
The invention discloses a reference product for detecting pathogenic microorganisms of bloodstream infection and a preparation method thereof, comprising the following steps: (1) obtaining human-derived cells and genomic DNAs of pathogenic microorganisms respectively; Genomic DNA fragmentation of source cells and pathogenic microorganisms; (3) Recovery and purification of fragmented DNA; (4) Valuation and mixing of fragmented DNA; (5) Mixing of fragmented DNA and plasma; (6) Detection of bloodstream infection Extraction and quality inspection of the reference product; using the above preparation method to prepare a reference product for the detection of pathogenic microorganisms of bloodstream infection. The source of the reference product is stable, close to natural plasma samples, covering various pathogens, and the value is more accurate. It can evaluate the quality of the detection results of bloodstream infection detection based on high-throughput sequencing, and can be used for different sequencing platforms, detection processes, Comparison of results between sampling methods, and development of technical standards for bloodstream infection detection based on high-throughput sequencing.
Owner:湖南赛哲智造科技有限公司
Method and device for identifying pathogenic influence of concurrent bacteria infection against poultry influenza virus infection
InactiveCN1204264CHighly pathogenicAchieve infectionMicrobiological testing/measurementTissue/virus culture apparatusBacteroidesSubtilisin
The invention relates to a method for identifying the influence of concurrent bacterial infection on the pathogenicity of avian influenza virus infection and a device used therefor. Co-inoculate bacteria that secrete subtilisin-like protease or / and bacteria that secrete tryptase and low-pathogenic avian influenza virus in the CEF culture, so that the bacteria cannot directly contact CPE but the enzymes they secrete can enter the culture medium, continue Cultivate and observe the presence or absence of CPE; according to the appearance of CPE, the cause of a large number of chicken deaths can be identified. The cell in vitro homogeneous culture device used in the method is composed of a cell culture bottle and a bacteria culture room, and the bacteria culture room is a closed structure with a microporous filter membrane with a pore size not greater than 0.22 microns that can communicate with the cell culture bottle. The method of the invention is simple and easy to operate, the identification time is short, the result is accurate and reliable, and the device used is low in price, and has important significance for the detection and prevention of bird flu.
Owner:SUN YAT SEN UNIV
A kind of influenza A virus subtyping detection method
ActiveCN108977585BQuick checkSensitive detectionMicrobiological testing/measurementForward primerHypotype
The invention discloses a subtype detection method of influenza A virus, which comprises the following steps: Step 1, designing and synthesizing primers; Step 2, preparing carboxyl magnetic beads connected with magnetic beads and forward primers; Step 3, A Influenza virus RNA extraction; Step 4, RT‑PCR; Step 5, magnetic adsorption and washing; Step 6, detection. The influenza A virus subtype detection method of the present invention can perform high-throughput, rapid, sensitive and specific typing of the influenza A virus subtype, and has good application value.
Owner:宁波怡和医药科技有限公司
Multi-gene detection method of Listeria monocytogenes based on quantum dot/graphene oxide nanometer platform
InactiveCN103233078BTo achieve the purpose of detecting multiple genes of Listeria monocytogenesImprove efficiencyMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceQuantum dot
The invention discloses a multi-gene detection method of Listeria monocytogenes based on a quantum dot / graphene oxide nanometer platform and belongs to the technical field of gene nanometer detection. The multi-gene detection method of Listeria monocytogenes comprises the following steps of: selecting two or more than two genes of the Listeria monocytogenes as target points of genetic detection and designing two pairs or more than two pairs of genetic PCR (Polymerase Chain Reaction) amplimers according to the analysis result of a gene conserved area; amplifying two or more than two genetic special sequences at the same time according to the principle of LATE-PCR to obtain a corresponding single stranded amplification product; then, hybridizing the single stranded amplification product with a quantum dot fluorescence probe; and finally, quenching and removing the non-hybridized quantum dot probe by using graphene oxide. When the target gene does not exist, all quantum dot fluorescence probes are quenched. On the contrary, when the target gene exists, corresponding fluorescent signals are obtained. The detection method has high detection reliability to Listeria monocytogenes.
Owner:SOUTH CHINA NORMAL UNIVERSITY
A strain of Beauveria bassiana and its application in controlling southern blueberry grubs
The invention provides a strain of Beauveria bassiana efficiently parasitizing grubs, a cultivation method of the strain and an application of the Beauveria bassiana strain in preventing and treating southern blueberry grubs. The Beauveria bassiana strain was deposited in the General Microorganism Center (CGMCC) of the China Committee for the Collection of Microbial Cultures (CGMCC) on December 29, 2017. The preservation number is: CGMCC NO.15090. It is specially used to control blueberry grub pests and has strong toxicity. , Insecticidal speed and so on.
Owner:GUIZHOU INST OF BIOLOGY
Reference product for detecting pathogenic microorganisms of bloodstream infection and preparation method thereof
ActiveCN113249441AReliable sourceHighly pathogenicMicrobiological testing/measurementEnzyme digestionReference product
The invention discloses a reference product for detecting pathogenic microorganisms of bloodstream infection and a preparation method thereof. The preparation method comprises the following steps: (1) respectively obtaining genomic DNAs (Deoxyribose Nucleic Acid) of human cells and pathogenic microorganisms; (2) fragmenting the genomic DNAs of human cells and pathogenic microorganisms by using an enzyme digestion method; (3) recovering and purifying the fragmented DNA; (4) valuing and mixing the fragmented DNA; (5) mixing the fragmented DNA and the plasma; and (6) extraction and quality inspection of the reference product for detecting bloodstream infection. The reference product for detecting the pathogenic microorganisms of bloodstream infection is prepared by using the preparation method. The reference product has a stable source, is close to a natural plasma sample, covers various pathogens, is more accurate in valuing, can be used for performing quality evaluation on a detection result of bloodstream infection detection based on high-throughput sequencing, can be used for comparing results of different sequencing platforms, detection processes and sampling modes, and formulating a technical standard for bloodstream infection detection based on the high-throughput sequencing.
Owner:湖南赛哲智造科技有限公司
Method of biochip for simultaneous testing avian influenza infection of human and fowls
InactiveCN101000340BRapid diagnosisHighly pathogenicBiological testingHighly pathogenicEpidemiologic survey
A biochip for detecting avain fluenza virus of both human and fowls is prepared as providing activated chip substrate, providing various fluenza virus subset sections to be sample-set, providing quality control protein, carrying out arrangement pattern design of point measurement, making sample-sets of quality control protein and various avain fluenza virus subset sections according to prepared pattern for detecting out antibodies generated by various subsets simultaneously.
Owner:深圳市赛尔生物技术有限公司
Riemerella anatipestifer (RA) and application thereof
ActiveCN103007261BHighly pathogenicAntibacterial agentsBacterial antigen ingredientsPerihepatitisHighly pathogenic
The invention relates to riemerella anatipestifer (RA). The collection number of the RA is CGMCC No.6832. The RA is used for preparing an RA inactivated vaccine. The prepared vaccine can prevent occurrence of infectious serositis of duck characterized by neurological symptom, fibrinous pericarditis, perihepatitis and air sacculitis. The number of RA 6832 in the inactivated vaccine is not less than 1*10<8>CFU / mL. The RA obtained by screening has high pathogenicity. The vaccine prepared from the RA can prevent occurrence of infectious serositis of duck characterized by neurological symptom, fibrinous pericarditis, perihepatitis and air sacculitis, and has good market promotion prospects.
Owner:QINGDAO VLAND BIOTECH INC +1
Method for preparing high efficiency biological weed control bacterial agent and its usage
InactiveCN1234277CLoose requirementsPlay the role of enriching soil microorganismsBiocideAnimal repellantsBiotechnologyBacterial strain
A process for preparing the efficient bacterial herbicide includes sampling the soil near the root of weeds such as crabgrass herb, caper euphorbia, cassia, etc. or their stem or leaves, separating with NPC culture medium, and the chlorella pyrenoidosa and glutamine synthetase depressants, and screening the bacterial strains with high herbiciding activity and broad spectrum. Its advantage is high herbiciding effect.
Owner:ZHEJIANG UNIV
High efficiency biological weed control bacterial and breeding selection method
InactiveCN1227979CLoose requirementsPlay the role of enriching soil microorganismsBiocideAnimal repellantsXanthomonas campestrisDepressant
An efficient bioherbiciding xanthomonas campestris pv. retroflexus is prepared through sampling the soil near the root of weeds such as crabgrass herb, caper euphorbia, cassia, etc and their stem and leaves, separating with NPC culture medium and chlorella pyrenoidosa and glutamine synthetase depressants, and screening the bacterial strains with high herbiciding activity and broad spectrum. Its advantage is high herbiciding effect.
Owner:ZHEJIANG UNIV
Sclerotium rolfsii solid fermentation culture medium and application thereof
The invention discloses a sclerotium rolfsii solid fermentation culture medium and an application thereof. The solid fermentation culture medium comprises a solid substrate and water, wherein the solid substrate comprises wheat bran and agricultural wastes, the wheat bran accounts for 20%-60% of the total weight of the solid substrate, and the weight ratio of the water to the solid substrate is (20-45):100; and the agricultural wastes select rice husk, sweet sorghum straw, an organic fertilizer, a wheat bran substrate, vinegar residue, wine lees, garden dead twigs or sawdust. The sclerotium rolfsii solid fermentation culture medium prepared by the preparation method disclosed by the invention is low in cost, the preparation process is simple, and a herbicide prepared by adopting the solid fermentation culture medium can be preserved at room temperature.
Owner:NANJING AGRICULTURAL UNIVERSITY
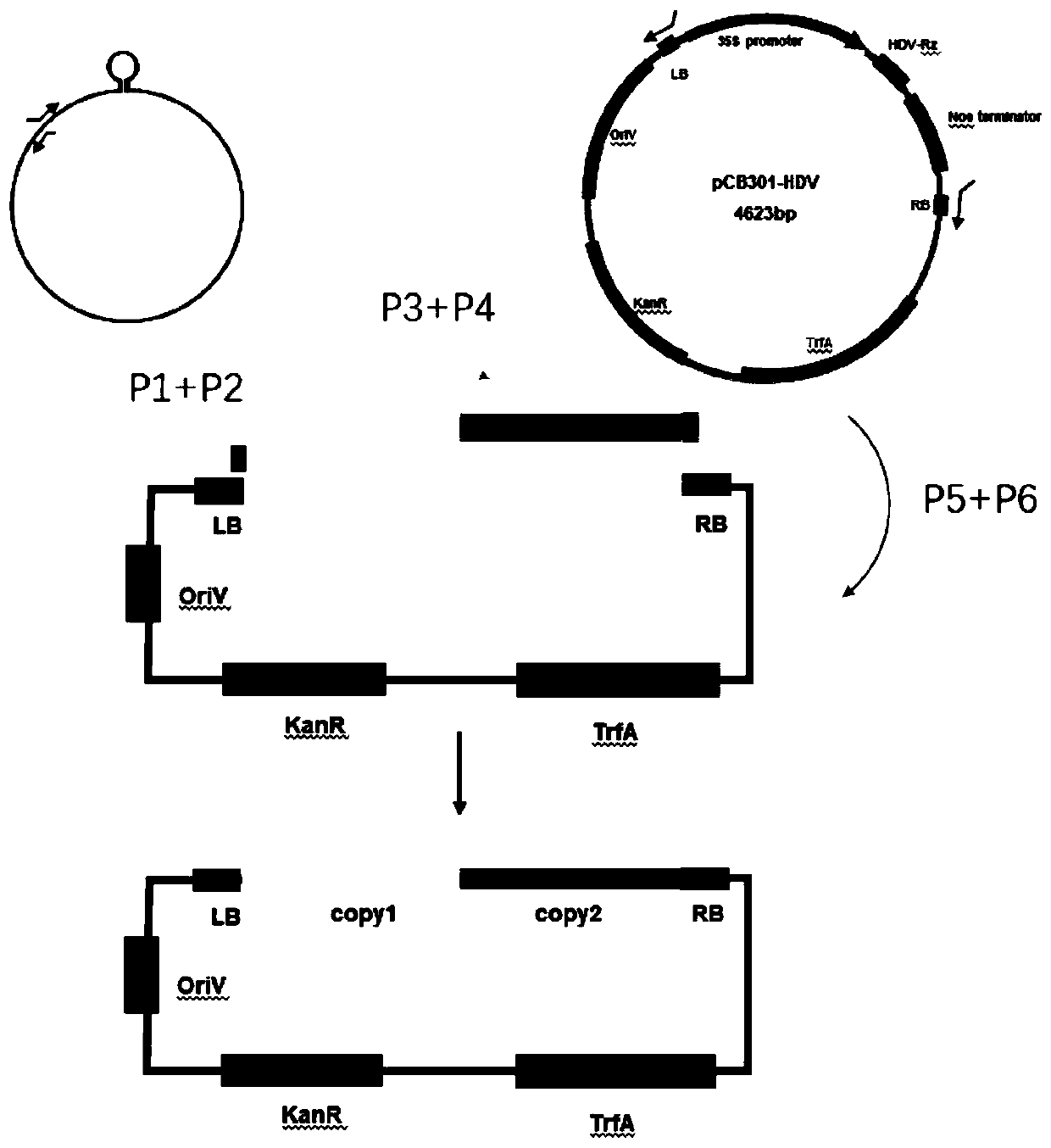
Construction method of infectivity clones for black nightshade curtoviruses
InactiveCN110904136AImprove fidelityHighly pathogenicBacteriaMicrobiological testing/measurementMolecular biologyEngineering
The invention provides a construction method of infectivity clones for black nightshade curtoviruses. The method comprises the steps of respectively designing primers P1 and P2, and P3 and P4, using pLB-NCTV as formwork, and performing PCR amplification of a first copy segment of NCTV and a second cope segment of NCTV; designing primers P5 and P6, using a pCB301 carrier as a formwork, performing PCR, and using the product as a carrier framework of NCTV infectivity clones; and constructing the first copy fragment and the second copy fragment by a multi-segment homologous recombination techniqueto the carrier framework to obtain the infectivity clones containing two copy fragments of NCTV. A construction system of infectivity clones for black nightshade curtoviruses is established, so thatthe high fidelity of a virus sequence is guaranteed, the pathogenicity is high, the construction success rate of the virus infectivity clones is high, and the infection efficiency is high. Besides, the invention further provides a black nightshade curtoviruses freeze-dry positive sample, and the method has important significance in further research on the black nightshade curtoviruses.
Owner:CHINA JILIANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com