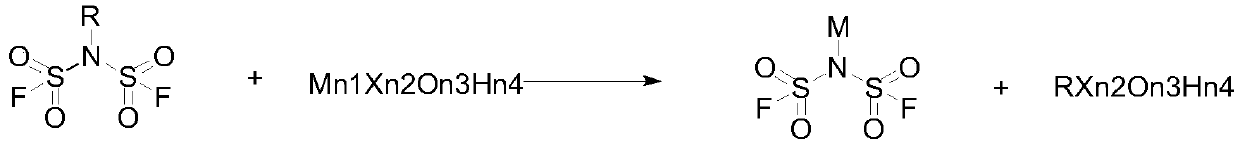
A new process for bisfluorosulfonyl imide salt
The technology of bisfluorosulfonimide salt and bisfluorosulfonimide is applied in the field of preparation of bisfluorosulfonimide salt, which can solve the problems of difficult separation of products, high industrial difficulty of LiFSI, complicated reaction process, etc. The three wastes are less, the implementation value is large, the social and economic benefits, and the properties are stable.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] In a specific embodiment, the preparation method of the bisfluorosulfonimide salt is characterized in that it comprises the following steps:
[0033] S1: The N-alkyl substituted bisfluorosulfonimide and salt are reacted in a solvent system to obtain a crude product;
[0034] S2: Crystallize and dry to obtain bisfluorosulfonimide salt.
[0035] The use of N-alkyl substituted bisfluorosulfonimides can make the system neutral during the reaction process without generating corrosive substances.
[0036] In a specific embodiment, the molar ratio of the N-alkyl substituted bisfluorosulfonimide to the salt is (1:1) to (1:3).
[0037] In a preferred embodiment, the molar ratio of the N-alkyl substituted bisfluorosulfonimide to the salt is 1:1.5.
[0038] By choosing the right salt and controlling the ratio of the two, it is possible to ensure that there are fewer three wastes in the production process, and there are more salts to choose from, which is suitable for large-scale production. ...
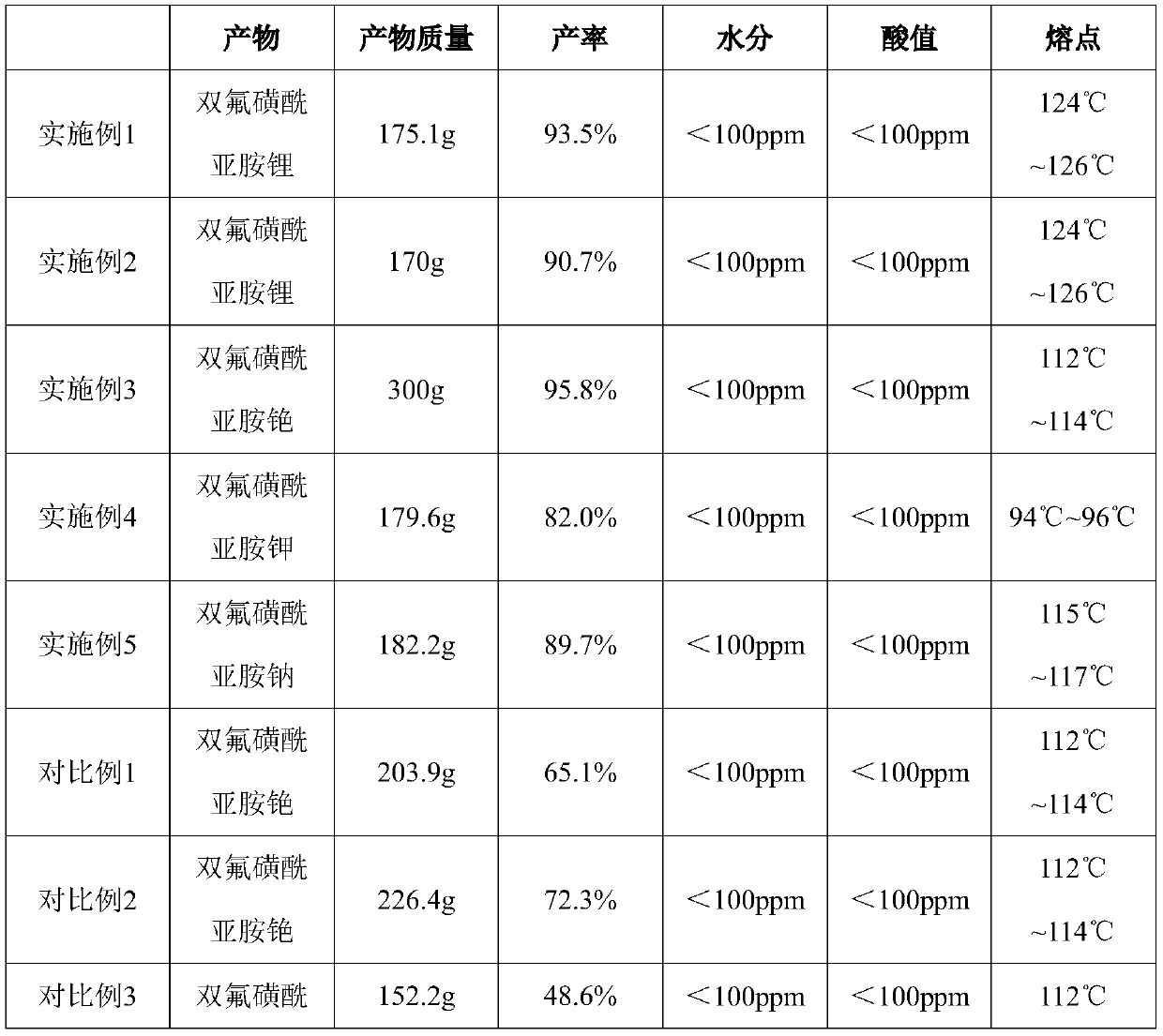
Embodiment 1
[0055] Embodiment 1 provides a preparation method of bisfluorosulfonimide salt, including the following steps:
[0056] Take 42.3 grams of anhydrous lithium chloride (dry at 105°C for 2 hours) and add it to 800 grams of dimethyl carbonate, cool to 25°C, and add 280 grams of N-benzyl-bisfluorosulfonimide dropwise, which takes 2 hours. After 5 hours, the reaction was continued at 25°C and 101KPa for 5 hours, then filtered, concentrated under reduced pressure to a thick state, 400 grams of toluene was added dropwise, stirred at 25°C for 2 hours, filtered, washed with toluene, and the filter cake was vacuum dried at 50°C. Lithium bisfluorosulfonimide.
Embodiment 2
[0058] Embodiment 2 provides a preparation method of bisfluorosulfonimide salt, including the following steps:
[0059] Take 26.0 g of anhydrous lithium fluoride, add it to 400 g of n-butyl acetate, control the temperature at about 50°C, add 200 g of N-methyl-bisfluorosulfonimide dropwise, which takes 2 hours, and then heat it at 50°C. , Continue the reaction for 2 hours at 101KPa, then hot filter, concentrate under reduced pressure to thick, dropwise add 600 grams of dichloromethane, stir at 15°C for 2 hours, filter, wash with dichloromethane, and dry the filter cake under vacuum at 60°C to obtain double Lithium fluorosulfonimide.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com