Metal complex, preparation method and application of metal complex to heavy metal hazard control
A technology for metal complexes and heavy metals, which can be used in applications, pharmaceutical formulations, fertilizer mixtures, etc., and can solve problems such as low heavy metal removal effect and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
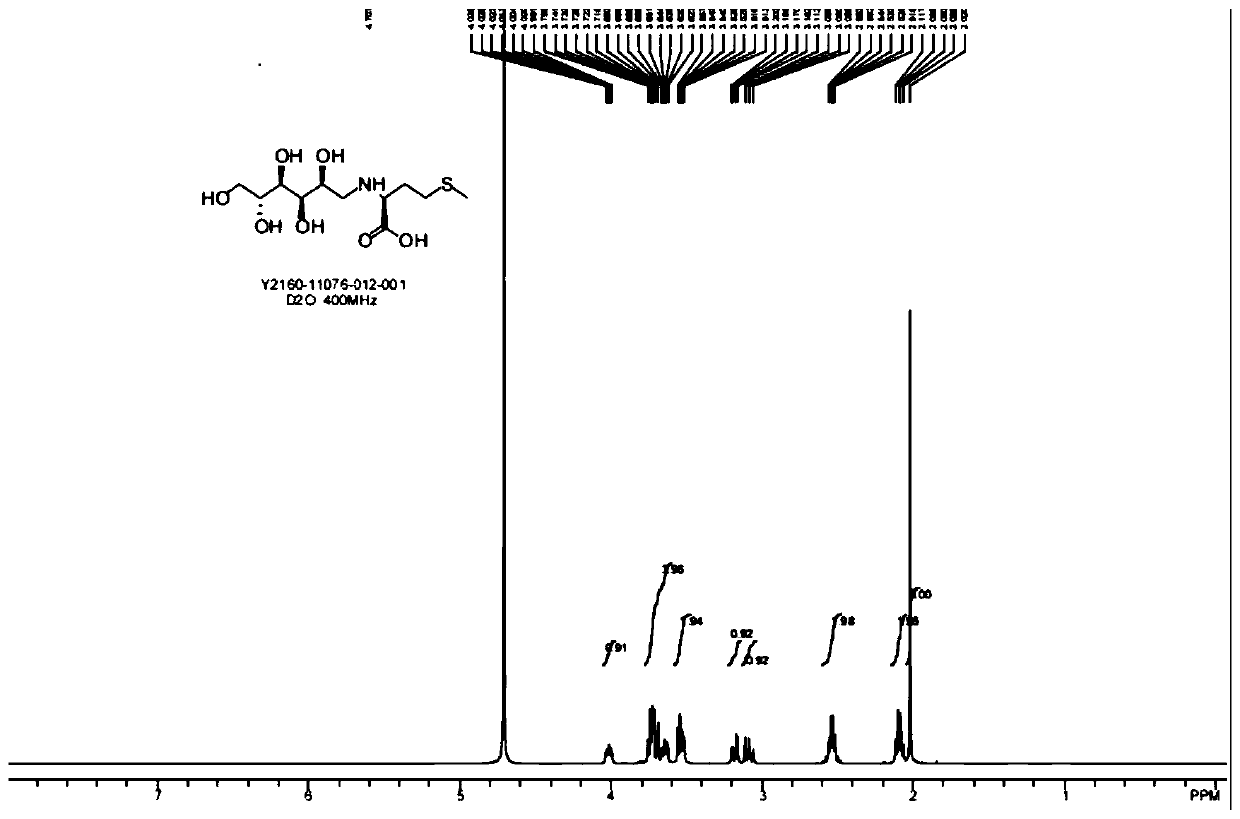
[0040] The present embodiment is the specific embodiment of preparing D4, and the structural formula of D4 is:
[0041]
[0042] Its preparation method is specifically:
[0043] S1:
[0044] In a 500mL three-necked flask, add 100mL of purified water, continue to add 24 g of NaOH into the three-necked flask, stir to dissolve, and then cool to room temperature.
[0045] N 2 Under protection, after adding 89.5g of L-methionine, stirred for 20 minutes, continued to add 90g of D-glucose, and stirred evenly. continue at N 2 Under protection, react at 50-60°C for 4 hours.
[0046] After the reaction is completed, cool to below 20°C, and add 46.2g of NaBH in batches during stirring 4 ; After addition, the temperature was raised to 25°C and stirred overnight.
[0047] Cool the reaction solution below 20°C, slowly add concentrated HCl dropwise to pH = 2, and remove B(OH) by suction filtration 3 , the filtrate was concentrated under reduced pressure. Continue to add 500ml of...
Embodiment 2
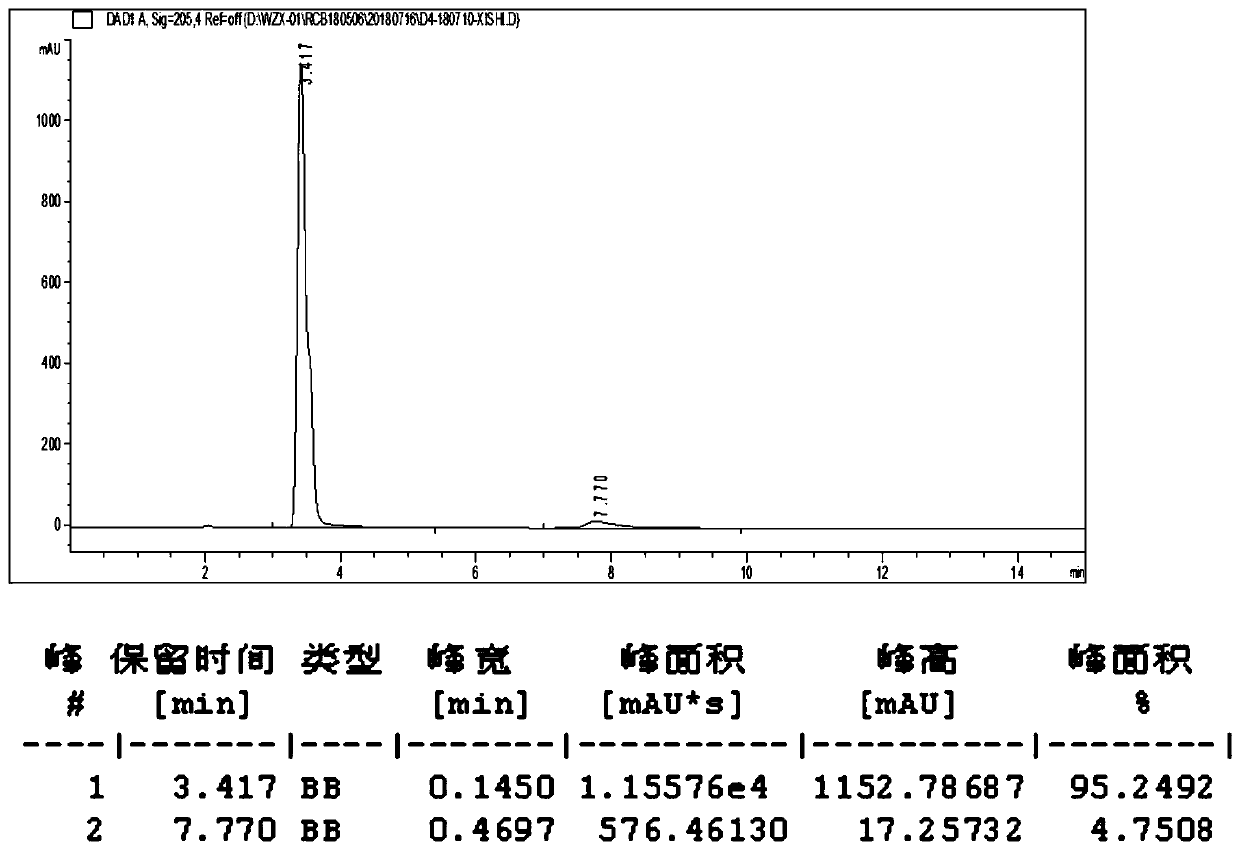
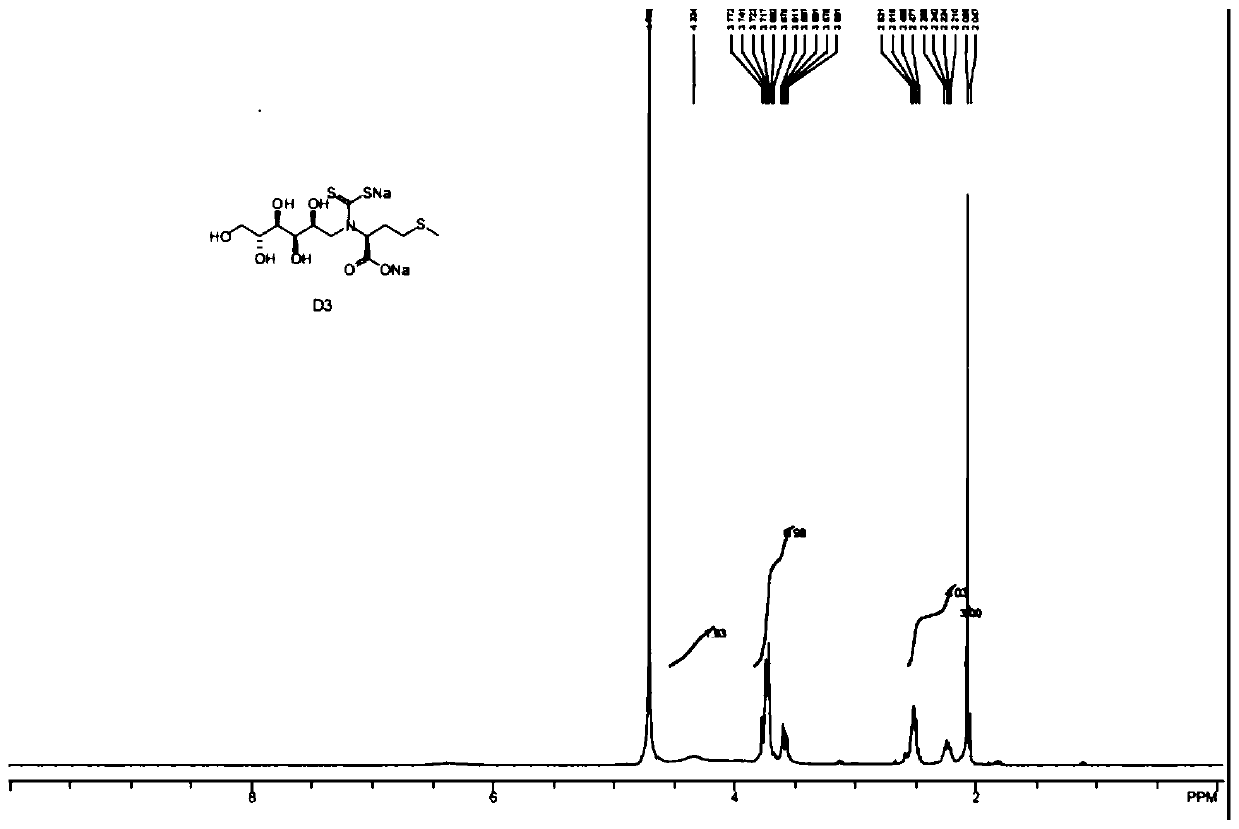
[0056] This example is a specific example of determining the stability of D4 prepared in Example 1.
[0057] Test samples: D4 and D3.
[0058] Inspection conditions: high temperature (25°C±2°C, 40°C±2°C), light (4500lux±500lux), high humidity (25°C±2°C, 75%RH±5%RH), in 5 days, 10 days, 30 days sampling test.
[0059] Test items
[0060]
[0061] Test Results
[0062] 1. Stored at a high temperature of 40°C±2°C for 10 days, the properties of D4 and D3, the clarity and color of the solution, and the acidity have almost no change compared with the 0 day; however, the water content has increased significantly compared with the 0 day. The total impurity of D3 increased from 2.89% to 15.67%, and the impurity increased significantly, so it can be seen that D3 is unstable under high temperature conditions and needs to be preserved at low temperature. The total impurity of D4 increased from 2.43% to 4.28%, and the impurity increased significantly, but the increase was much lower...
Embodiment 3
[0067] This example is a specific example of measuring the cadmium-dispelling effect of D4 prepared in Example 1.
[0068] Modeling: Animals in the model group were given a mixed solution of Cd and ME (Cd: 3.0 μmol / kg body weight, ME: 12 μmol / kg body weight) at a dose of 5 mL / kg body weight through the ear vein, injected once a day for 5 consecutive days modeling. 35 days after the last injection, collect and detect rabbit blood, 6h metabolic urine, detect blood cadmium and urine cadmium levels, and screen 48 model animals, half male and half male. Modeling standard: Compared with the blank control group, the urine Cd content of the model animals increased, and there was a statistically significant difference (P<0.01). It suggested that the rabbit model of chronic cadmium poisoning was established successfully. 48 model animals were screened.
[0069] Drug treatment: Animals in each group were infused with corresponding drugs through ear veins, and the infusion rate was con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com