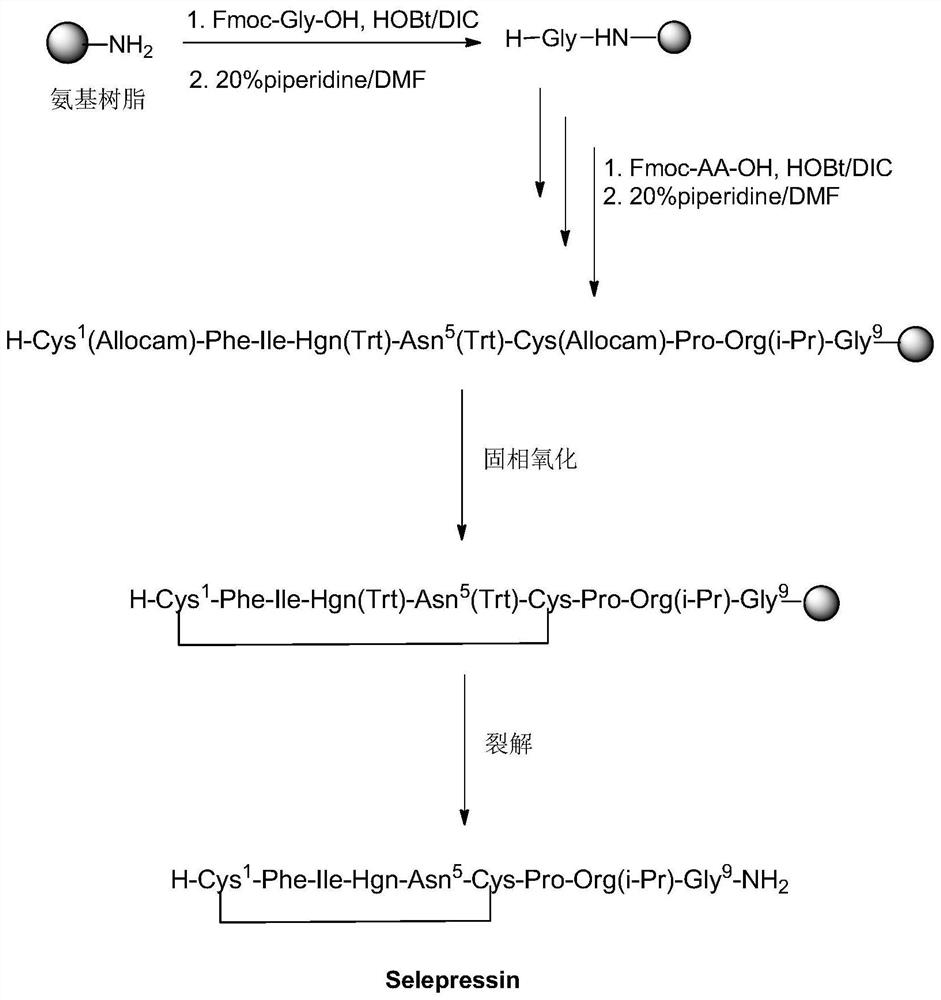
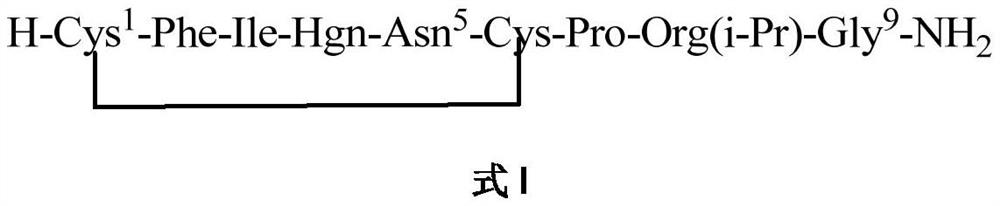
A method for solid-phase synthesis of vasopressin receptor peptide agonist selepressin
A technology of vasopressin and synthetic method, applied in the field of solid-phase synthesis of Selepressin, which can solve the problems of difficult removal of iodine, many impurities, low yield and purity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The preparation of embodiment 1 polypeptide resin
[0041] Weigh 50 g of Rink Amide resin with a substitution degree of 1.0 mmol / g into a solid-phase reaction column, add 50 mL of DMF, and swell with nitrogen gas for 60 minutes; then deprotect with 50 mL of DBLK for 6 min+8 min, and wash with 100 mL of DMF for 6 times. Weigh 74.3g (250mmol) Fmoc-Gly-OH and 33.8g (250mmol) HOBT and dissolve them with 150mL DMF, add 31.5g (210mmol) DIC under ice-water bath to activate for 3min, then add the mixed solution into the reaction column, and react at room temperature for 2 Hours, use ninhydrin to detect the end of the reaction (if the resin is colorless and transparent, the reaction is terminated; if the resin develops color, the reaction is extended for 1 hour). After the reaction is over, wash the resin with 150mL DMF for 3 times, add 150mL DBLK for deprotection for 6min + 8min, wash the resin with 150mLDMF for 6 times, and detect the color of the resin with ninhydrin. Repeat...
Embodiment 2
[0042] The preparation of embodiment 2 polypeptide resins
[0043]Weigh 50 g of Rink Amide-AM resin with a substitution degree of 1.0 mmol / g into a solid-phase reaction column, add 50 mL of DMF, and swell with nitrogen gas bubbling for 60 minutes; then deprotect with 50 mL of DBLK for 6 min+8 min, and wash with 100 mL of DMF for 6 times. Weigh 74.3g (250mmol) Fmoc-Gly-OH and 33.8g (250mmol) HOBT and dissolve them with 150mL DMF, add 31.5g (210mmol) DIC under ice-water bath to activate for 3min, then add the mixed solution into the reaction column, and react at room temperature for 2 Hours, use ninhydrin to detect the end of the reaction (if the resin is colorless and transparent, the reaction is terminated; if the resin develops color, the reaction is extended for 1 hour). After the reaction is over, wash the resin with 150mL DMF for 3 times, add 150mL DBLK for deprotection for 6min + 8min, wash the resin with 150mLDMF for 6 times, and detect the color of the resin with ninhyd...
Embodiment 3
[0044] The preparation of embodiment 3 polypeptide resins
[0045] Weigh 50 g of MBHA resin with a substitution degree of 1.0 mmol / g into a solid-phase reaction column, add 50 mL of DMF, and swell with nitrogen gas for 60 minutes; then deprotect with 50 mL of DBLK for 6 min + 8 min, and wash 6 times with 100 mL of DMF. Weigh 74.3g (250mmol) Fmoc-Gly-OH and 33.8g (250mmol) HOBT and dissolve them with 150mL DMF, add 31.5g (210mmol) DIC under ice-water bath to activate for 3min, then add the mixed solution into the reaction column, and react at room temperature for 2 Hours, use ninhydrin to detect the end of the reaction (if the resin is colorless and transparent, the reaction is terminated; if the resin develops color, the reaction is extended for 1 hour). After the reaction is over, wash the resin with 150mL DMF for 3 times, add 150mL DBLK for deprotection for 6min + 8min, wash the resin with 150mLDMF for 6 times, and detect the color of the resin with ninhydrin. Repeat the ab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com