Crosslinkable copolymer and application thereof
A cross-linked copolymer and monomer technology, applied in the directions of ester copolymer adhesives, adhesive types, adhesive additives, etc., to achieve the effect of prolonging construction time, less impact on peeling rate, and improving workability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
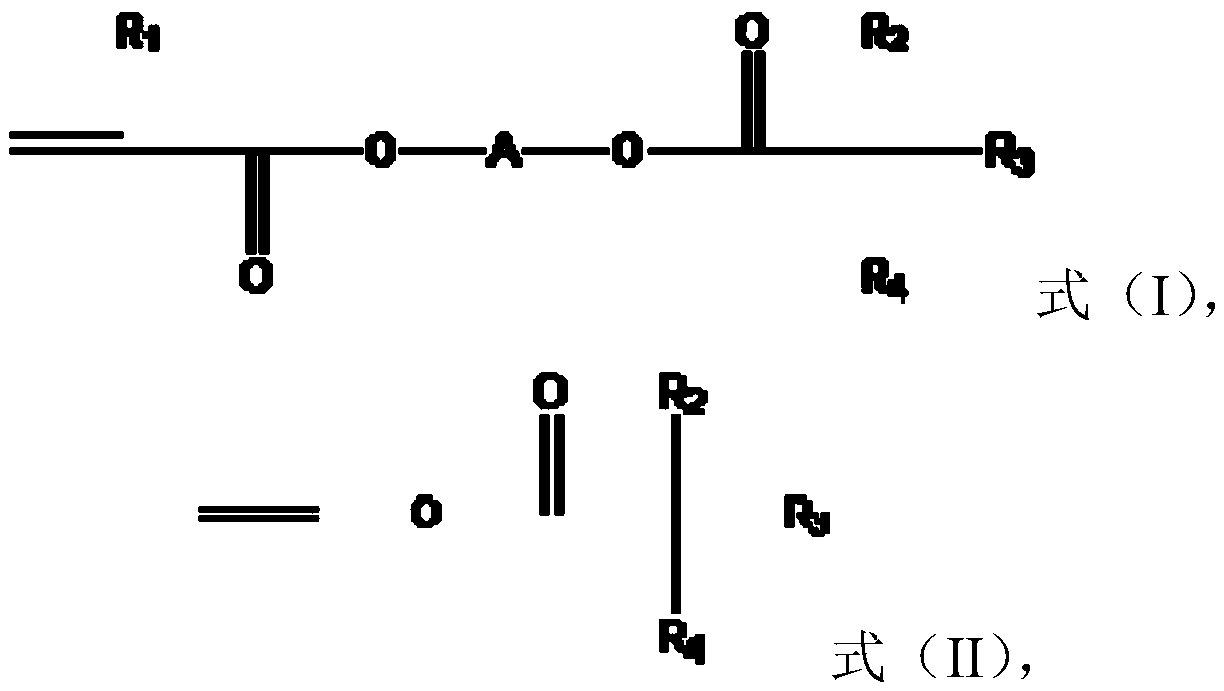
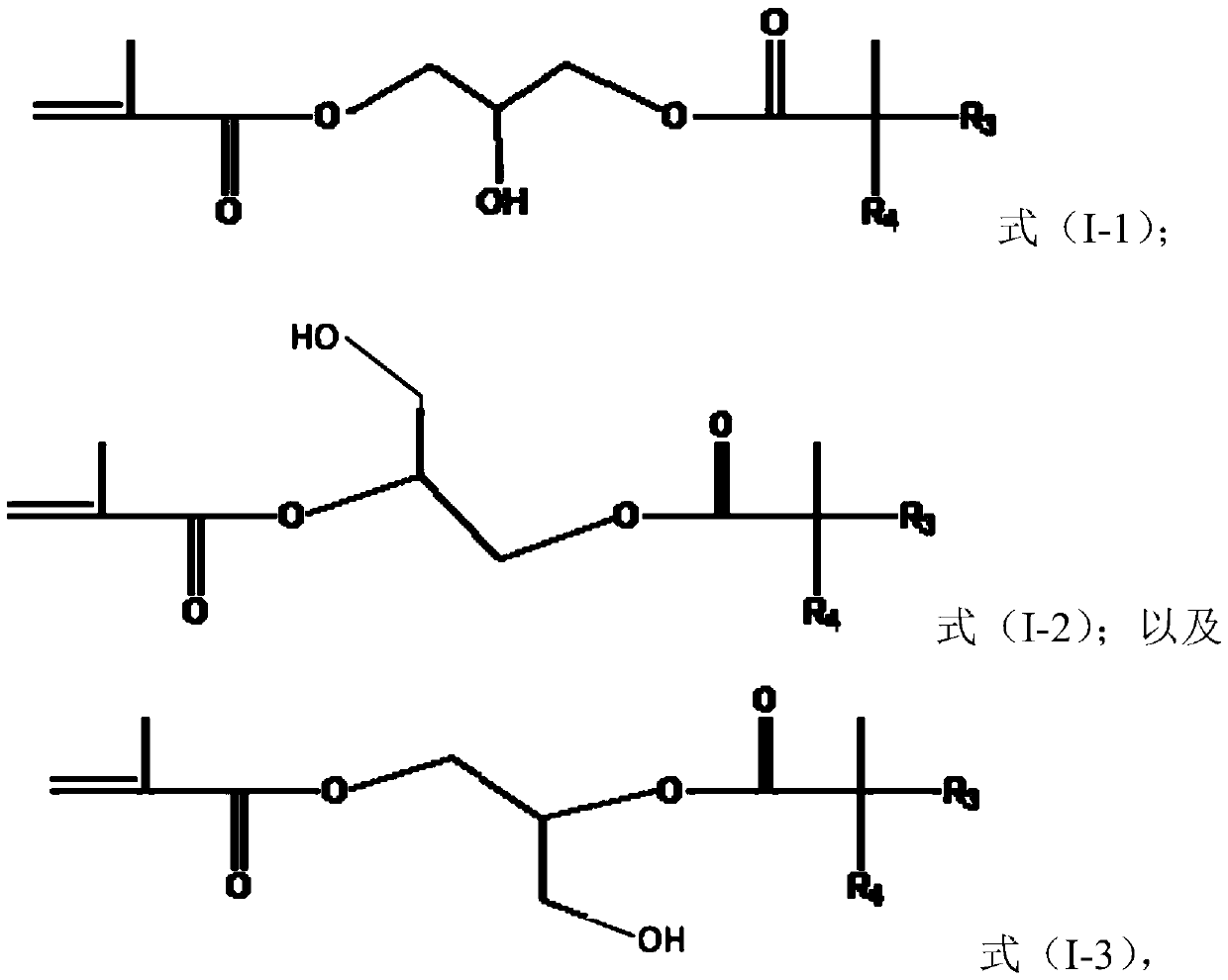
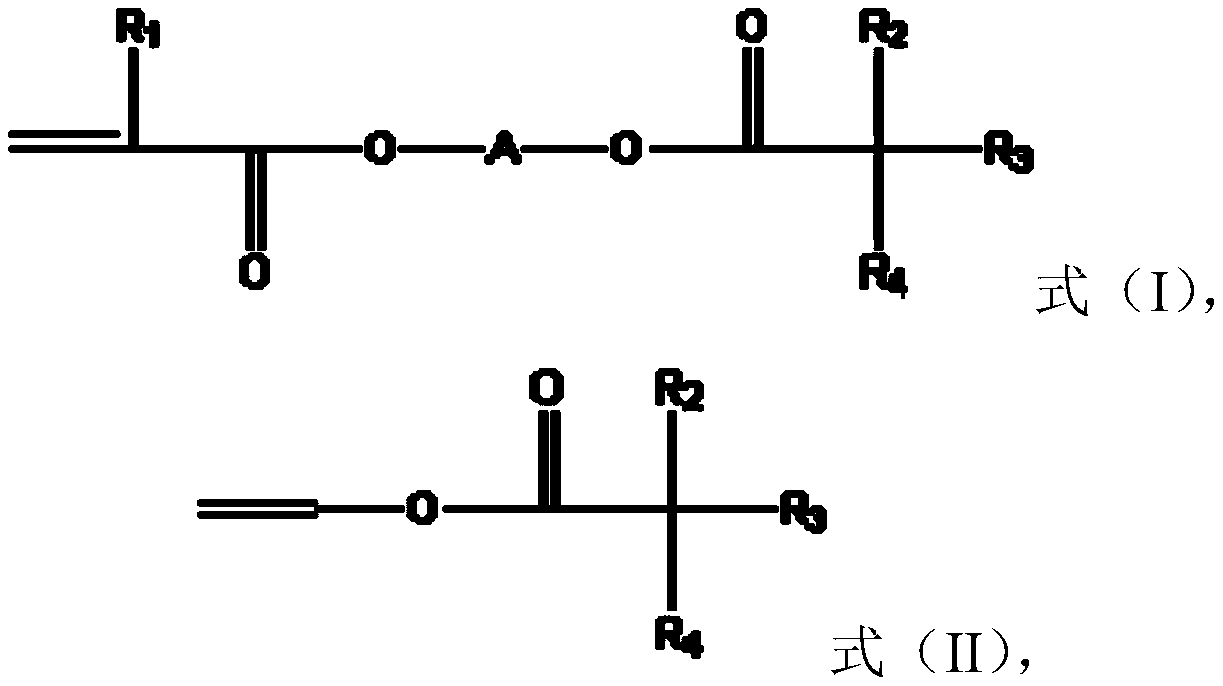
[0049] The preparation method of the monomer of formula (I) includes but is not limited to obtaining by making tertiary carbonic acid glycidyl esters (tertiary carbonic acid glycidyl esters) react with (meth)acrylic acid, for example, can be obtained by making C 5 to C 13 It is obtained by reacting glycidyl carboxylate with (meth)acrylic acid, or by reacting glycidyl (meth)acrylate with C 5 to C 13 New carboxylic acid reaction obtained. C 5 to C 13 Specific examples of glycidyl neocarboxylates include, but are not limited to, glycidyl pivalate, glycidyl neoheptanoate, glycidyl neononanoate, glycidyl neodecanoate, glycidyl neoundecanoate, and Glycidyl neotridecanoate. C 5 to C 13 Specific examples of neocarboxylic acids include, but are not limited to, neopentanoic acid, neoheptanoic acid, neononanoic acid, neodecanoic acid, neodecanoic acid, neodecanoic acid, and neotridecanoic acid.
[0050] The preparation of the monomer of formula (II) includes but not limited to th...
Embodiment approach
[0053] According to one embodiment of the present invention, monomer (B) may have the structure of the following formula (III):
[0054]
[0055] In formula (III), R 11 is H or methyl, and R 12 for C 1 to C 18 alkyl.
[0056] Specific examples of monomers of formula (III) include, but are not limited to, methyl acrylate, methyl methacrylate, ethyl acrylate, ethyl methacrylate, propyl acrylate, propyl methacrylate, isopropyl acrylate, Isopropyl methacrylate, n-butyl acrylate, n-butyl methacrylate, isobutyl acrylate, isobutyl methacrylate, secondary butyl acrylate, secondary butyl methacrylate, tertiary butyl acrylate , tertiary butyl methacrylate, n-pentyl acrylate, n-pentyl methacrylate, isopentyl acrylate, isopentyl methacrylate, n-hexyl acrylate, n-hexyl methacrylate, n-heptyl acrylate, methyl n-heptyl acrylate, n-octyl acrylate, n-octyl methacrylate, 2-ethylhexyl acrylate, 2-ethylhexyl methacrylate, isooctyl methacrylate, n-nonyl acrylate, methyl n-nyl acrylate, is...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Surface resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com