Ibrutinib salts, crystals, preparation method, pharmaceutical compositions and applications thereof
An ibrutinib salt and ibrutinib technology are applied in ibrutinib salt, its crystal, preparation, pharmaceutical composition and application fields, can solve problems such as lack, achieve improved hygroscopicity, simple preparation method, The effect of good industrial application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0044] Preparation Example 1 Preparation of Ibrutinib Hydrochloride Crystals
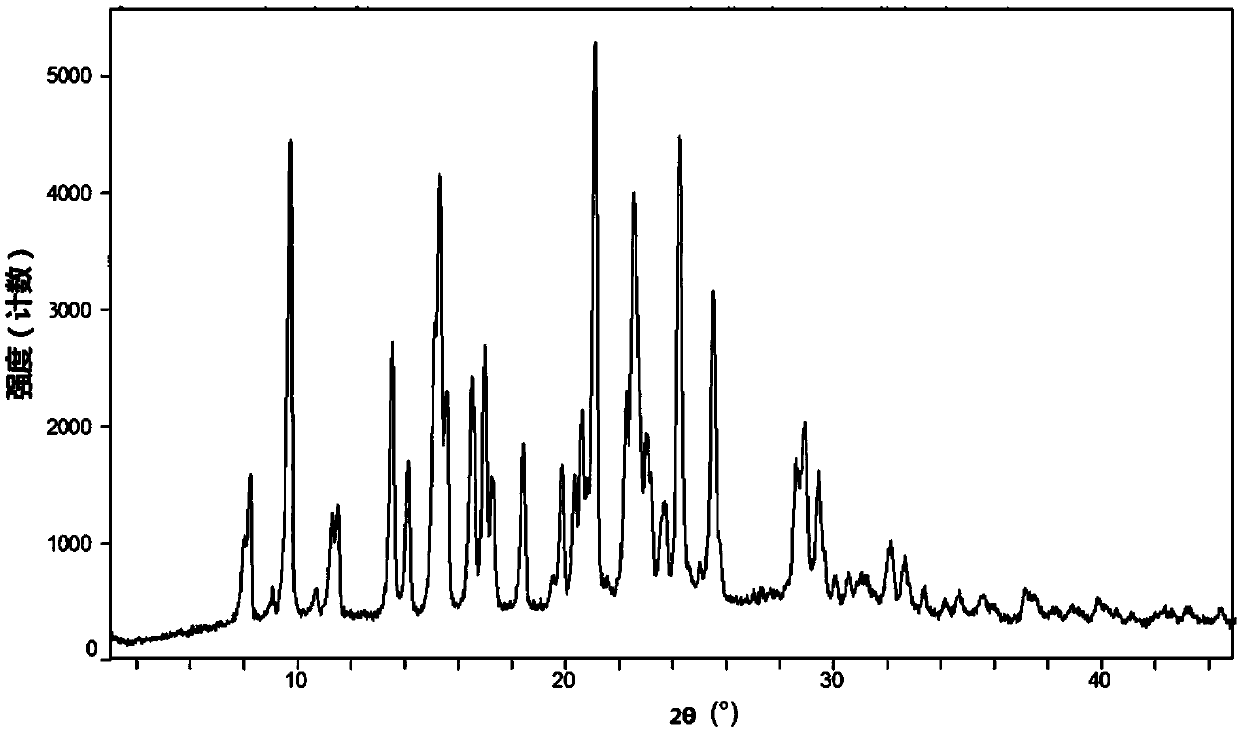
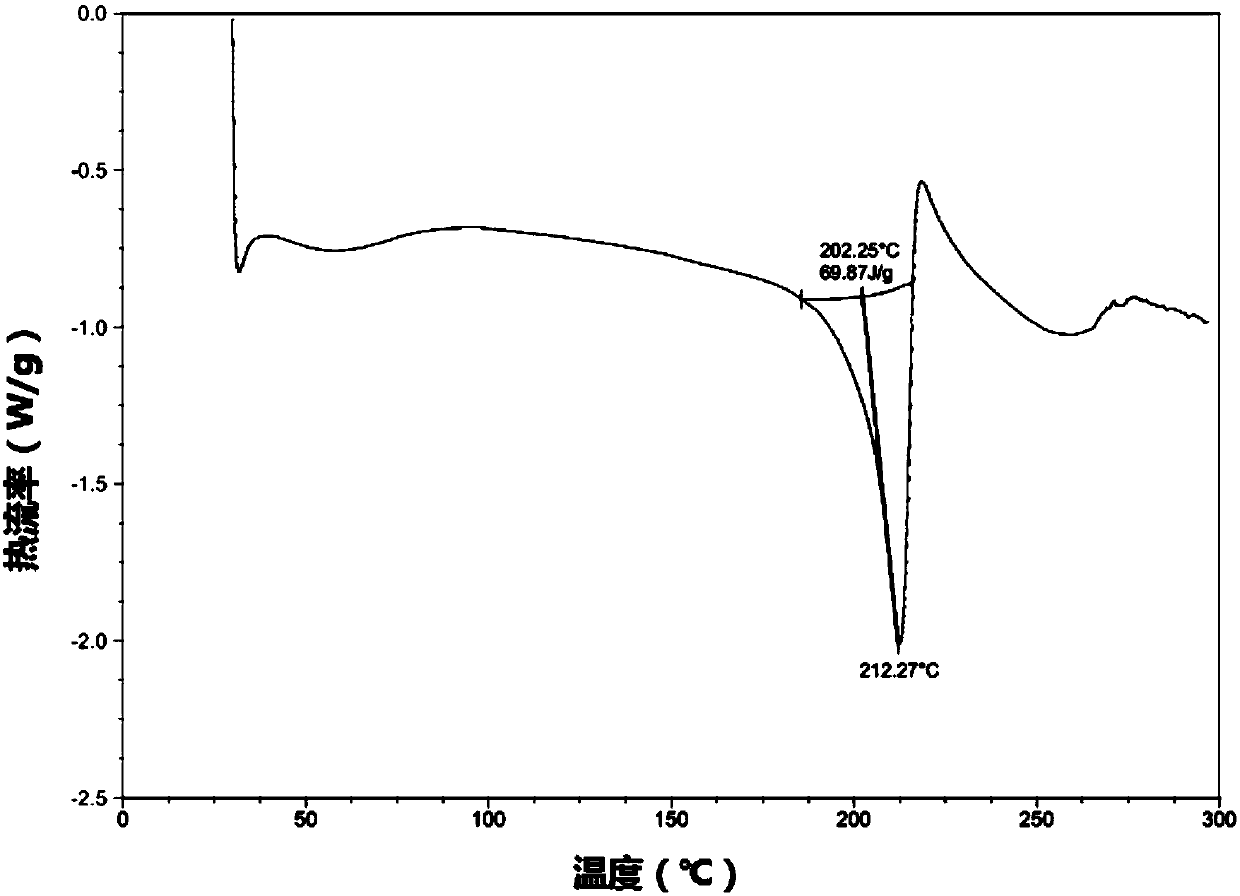
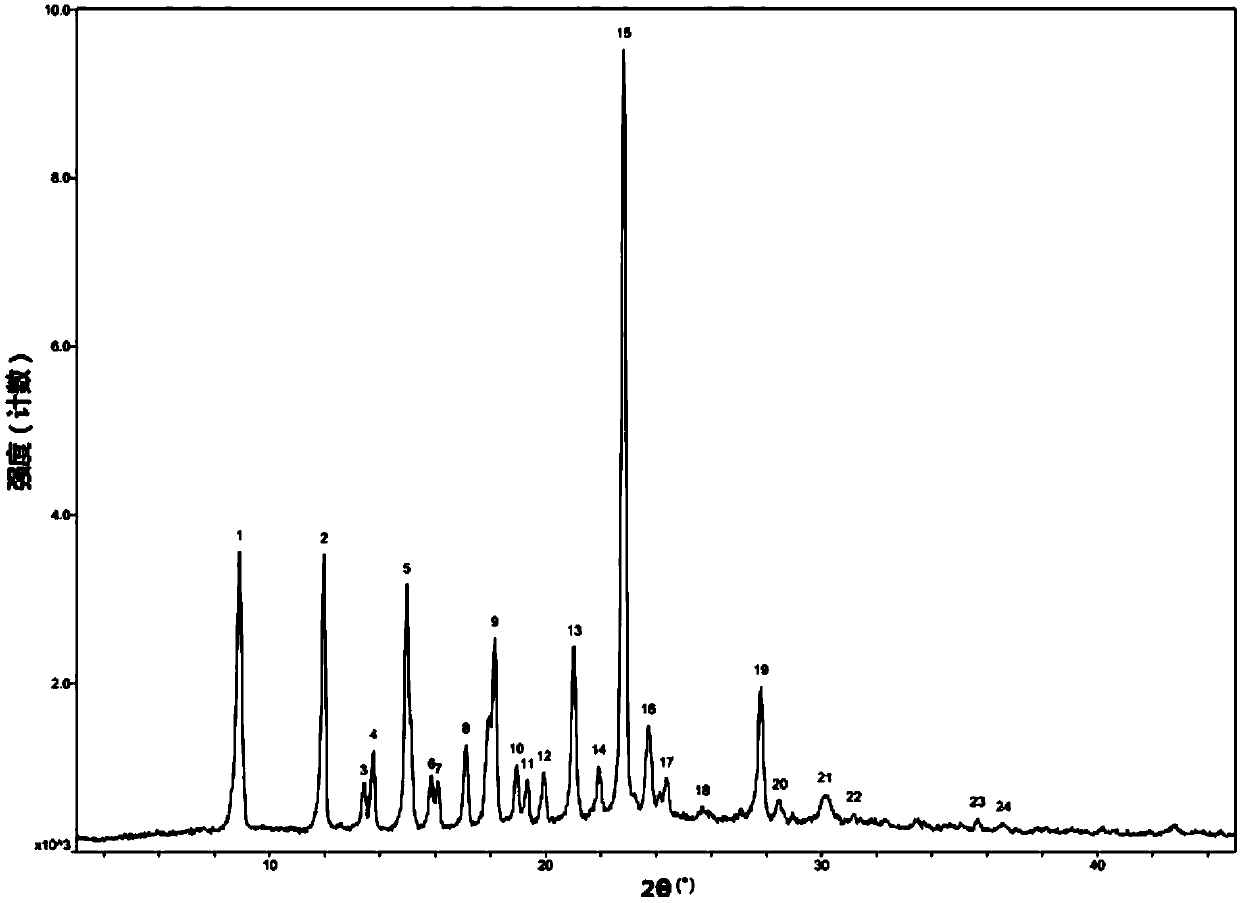
[0045] Add ibrutinib (2.0g, 4.5mmol) and methanol 4ml into a 100ml reaction bottle, add HCl methanol solution (2.25ml, 2.0M) dropwise under stirring, heat to about 50°C after dropping, and make the reaction solution Dissolved, cooled to 20°C and stirred for 4h. Filter, wash with a small amount of methanol, and dry under vacuum at 60°C for 6 hours to obtain ibrutinib hydrochloride crystals as a white crystalline powder, 1.4 g in total, yield 65%, M.P.: 214-216°C, HPLC content 99.5%. C 25 h 24 N 6 o 2 .HCl elemental analysis experimental value: C: 62.37, H: 5.38, N: 17.39; theoretical value C: 62.95, H: 5.28, N: 17.62, through X-ray powder diffraction (XRD) and differential thermal scanning calorimetry ( DSC) characterization and identification, the results are as follows Figure 1-2 shown.
preparation Embodiment 2
[0046] Preparation Example 2 Preparation of Ibrutinib Hydrochloride Crystals
[0047] Add ibrutinib (2.0g, 4.5mmol) and 10ml of ethanol to a 100ml reaction bottle, add HCl ethanol solution (2.75ml, 2.0M) dropwise under stirring, heat to about 80°C after dropping, and make the reaction solution Dissolved, cooled to 0°C and stirred for 4h. Filter, wash with a small amount of ethanol, and dry under vacuum at 60°C for 6 hours to obtain ibrutinib hydrochloride crystals as a white crystalline powder, totaling 1.6g, yield 74%, M.P.: 214-216°C, HPLC content 99.6%. The XRD and DSC characterization results are the same as in Example 1.
preparation Embodiment 3
[0048] Preparation Example 3 Preparation of Ibrutinib Hydrochloride Crystals
[0049] Add ibrutinib (2.0g, 4.5mmol) and 8ml of isopropanol to a 100ml reaction bottle, add HCl in isopropanol (3.4ml, 2.0M) dropwise with stirring, and heat to about 60°C after dropping , The reaction solution was dissolved, cooled to 0°C and stirred for 4h. Filter, wash with a small amount of isopropanol, and dry under vacuum at 60°C for 6 hours to obtain ibrutinib hydrochloride crystals as a white crystalline powder, totaling 1.5g, yield 69%, M.P.: 214-216°C, HPLC content 99.5%. The XRD and DSC characterization results are the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com