Timosaponin BII temperature/ion-sensitive nasal in-situ gel and application thereof
A timosaponin and ion-sensitive technology, applied in the field of medicine, can solve the problems of prevention and treatment of Alzheimer's disease, etc., and achieve the effect of overcoming short local residence time and significant preventive and therapeutic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Step 1. Weigh 0.3 parts of deacetylated gellan gum in a beaker by weight, add an appropriate amount of deionized water, fully swell in a hot water bath at 70°C, and cool at 4°C;
[0043] Step 2: Take another 20 parts of poloxamer 407 and 0.1 part of sodium alginate in parts by weight, and slowly add them to the swollen deacetylated gellan gum in step 1 while stirring, stir evenly, and stand at 4°C for 5 hours , to get the gel matrix;
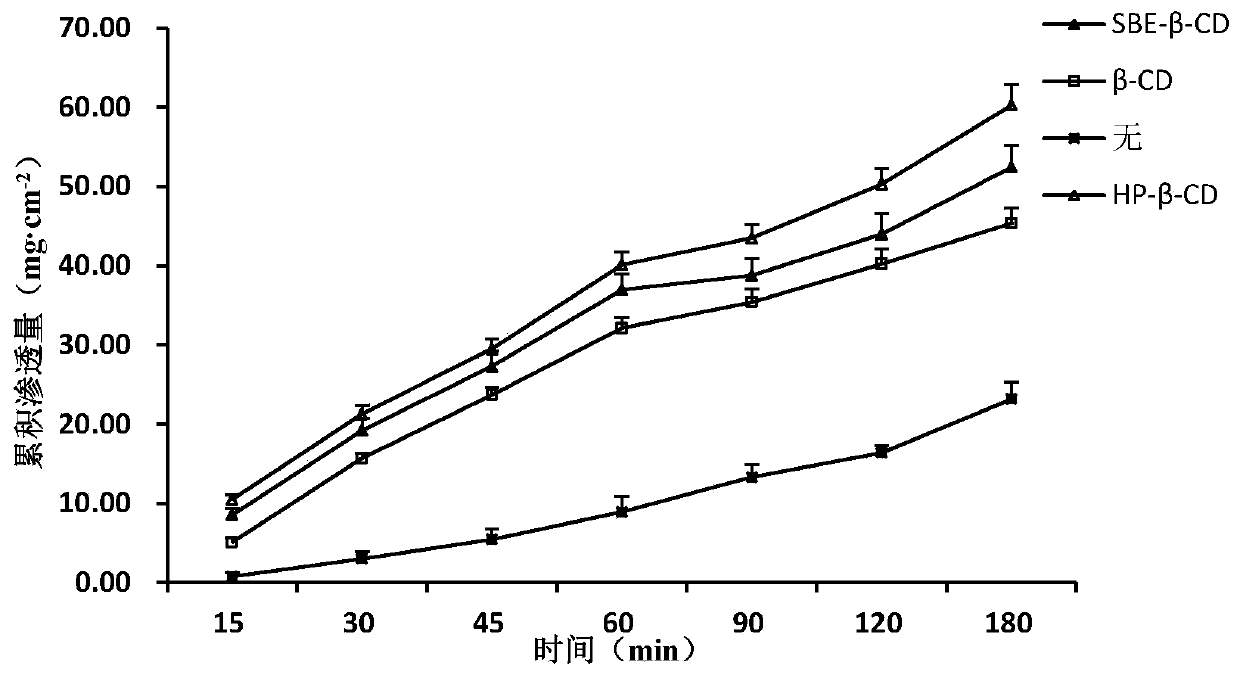
[0044] Step 3: Add 0.5 parts of chlorobutanol and 2.5 parts of hydroxypropyl-β-cyclodextrin to the gel matrix obtained in Step 2 while stirring to obtain a composite gel;
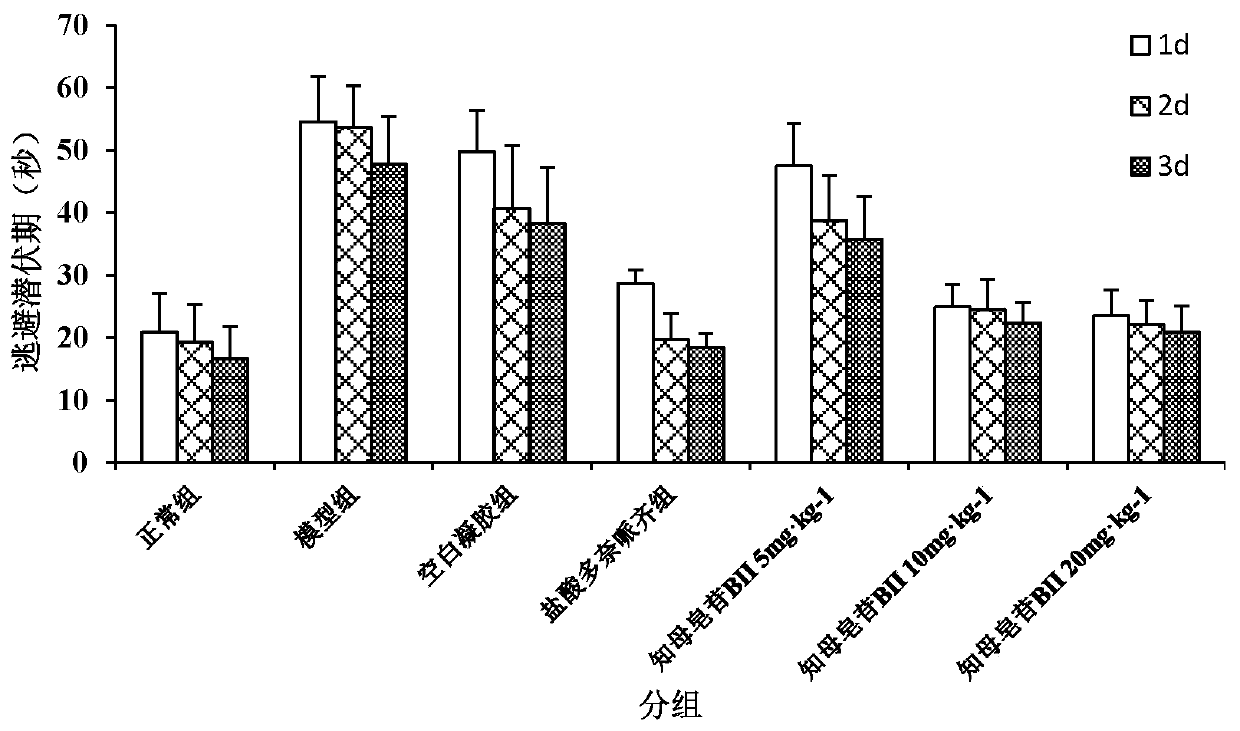
[0045] Step 4: Add 25 parts by weight of timosaponin BII to the composite gel obtained in step 3 under stirring to obtain the timosaponin BII temperature / ion-sensitive in-situ nasal gel.
Embodiment 2
[0047] Step 1. Weigh 0.1 part of deacetylated gellan gum in a beaker by weight, add an appropriate amount of deionized water, fully swell in a hot water bath at 80°C, and then cool at 4°C;
[0048] Step 2: Take another 16 parts of poloxamer 407 and 0.3 parts of sodium alginate in parts by weight, and slowly add them to the swollen deacetylated gellan gum in step 1 while stirring, stir evenly, and let stand at 4°C for 10 hours , to get the gel matrix;
[0049] Step 3: Add 0.5 parts of chlorobutanol and 2.5 parts of hydroxypropyl-β-cyclodextrin to the gel matrix obtained in Step 2 while stirring to obtain a composite gel;
[0050] Step 4: Add 5 parts by weight of timosaponin BII to the composite gel obtained in step 3 under stirring to obtain the timosaponin BII temperature / ion-sensitive in-situ nasal gel.
Embodiment 3
[0052] Step 1. Weigh 0.2 parts of deacetylated gellan gum in a beaker by weight, add an appropriate amount of deionized water, fully swell in a hot water bath at 70°C, and then cool at 4°C;
[0053]Step 2: Take another 18 parts of poloxamer 407 and 0.2 parts of sodium alginate in parts by weight, and slowly add them to the swollen deacetylated gellan gum in step 1 while stirring, stir evenly, and stand at 4°C for 5 hours , to get the gel matrix;
[0054] Step 3: Add 0.5 parts of chlorobutanol and 2.5 parts of hydroxypropyl-β-cyclodextrin to the gel matrix obtained in Step 2 while stirring to obtain a composite gel;
[0055] Step 4: Add 50 parts by weight of timosaponin BII to the composite gel obtained in step 3 under stirring to obtain the timosaponin BII temperature / ion-sensitive in-situ nasal gel.
PUM
| Property | Measurement | Unit |
|---|---|---|
| height | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com