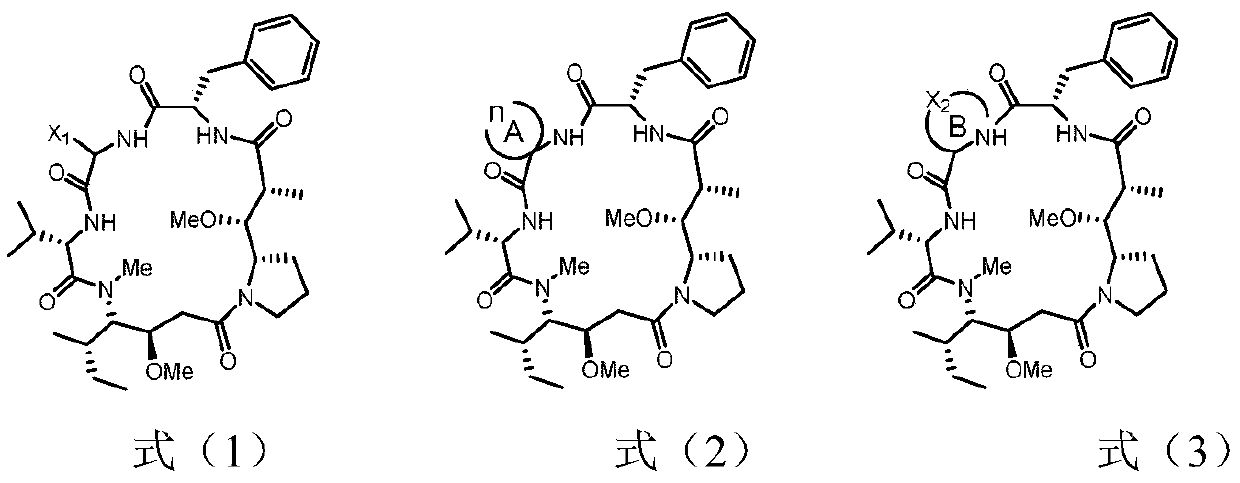
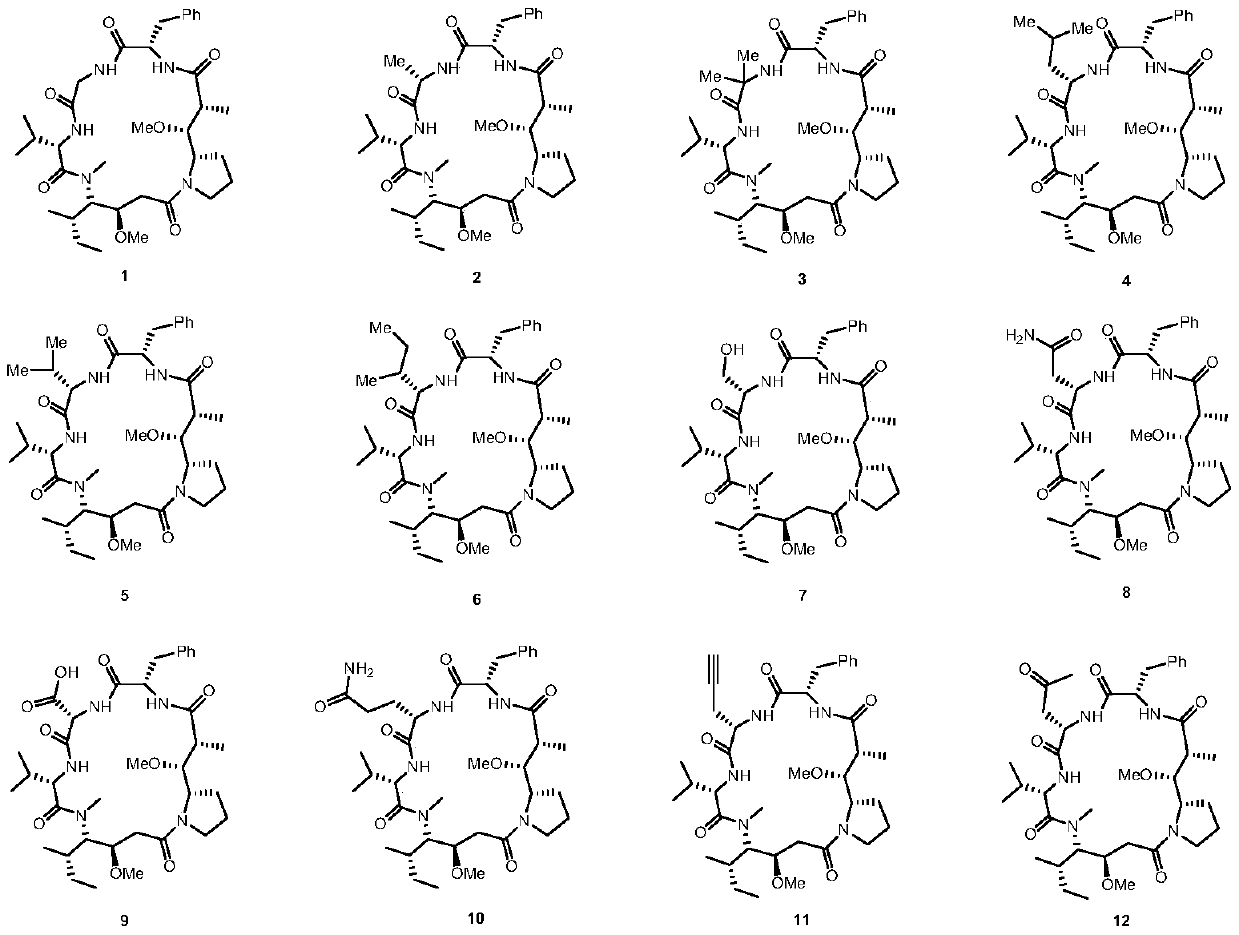
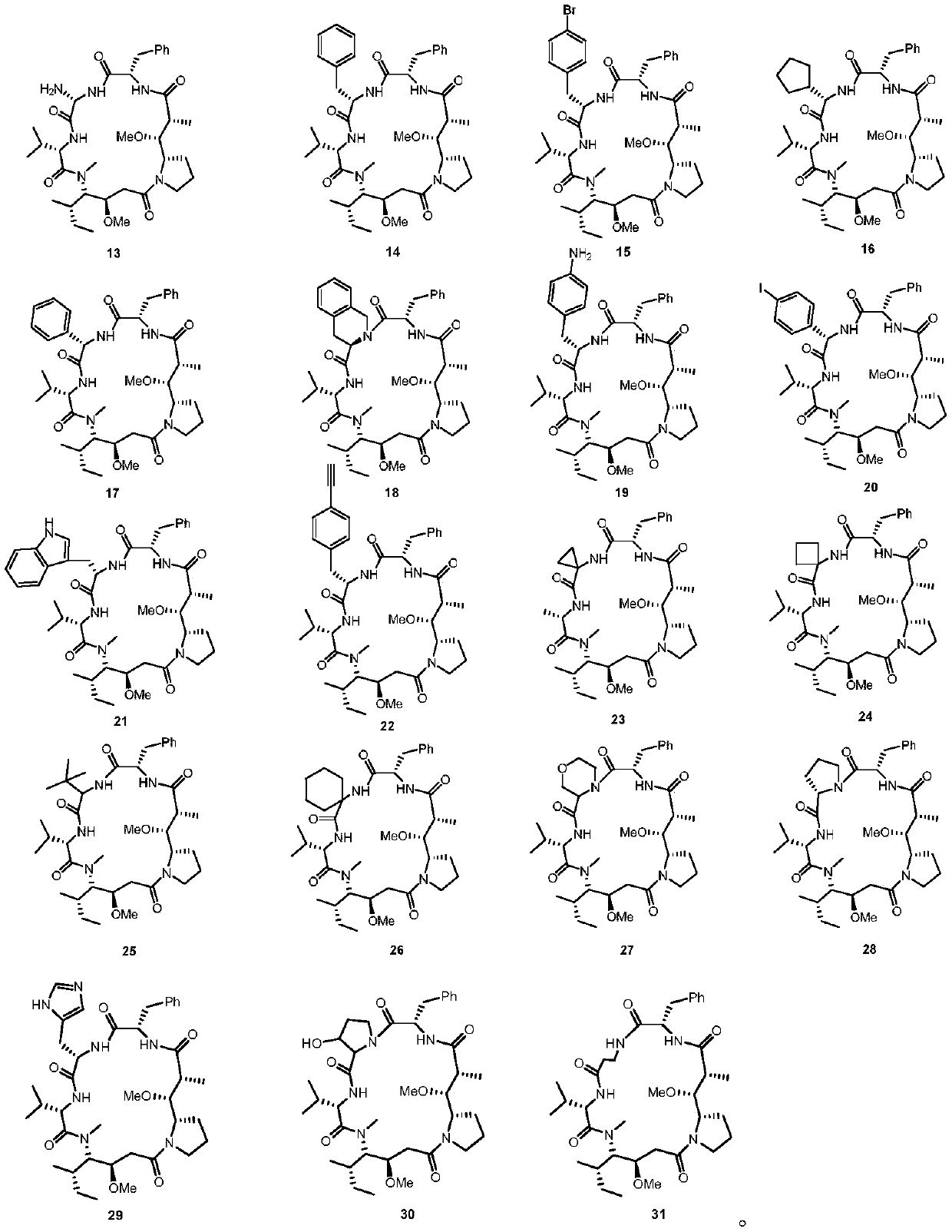
Dolastatin 10 cyclopeptide derivative and preparation method and application thereof
A technology of dolastatin and derivatives, applied in the direction of cyclic peptide components, peptides, drug combinations, etc., can solve the problems of poor stability, increased compound structure, high toxicity, etc., and achieve the effect of high stability, low toxicity, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Compound 1
[0039]
[0040] Weigh the linear pentapeptide and dissolve it in DMF (1×10 -3 mol / L), ice-water bath cooling, nitrogen protection, and then add DMAP (2 equivalents) in sequence, after the addition, keep the ice-water bath stirring for 5 minutes, then add EDCI (5 equivalents) at once, continue to maintain the ice-water bath reaction 1-2 After hours, the temperature is raised to room temperature and the reaction is about 6 hours until the LC-MS detects that the reaction is complete. Quench with water, extract with ethyl acetate, and wash the organic phase with 10% citric acid aqueous solution, water, saturated brine, and anhydrous Na 2 SO 4 Dry, spin off the solvent under reduced pressure to obtain the crude cyclic peptide. The crude product was separated and purified by preparative HPLC to obtain cyclic peptide compound 1, a white solid (10 mg, 47%). 1 H NMR(400MHz, DMSO-d 6 )δ7.41–7.05(m,5H), 5.43–5.23(m,1H), 4.92–4.65(m,1H), 4.47(dd,J=34.4,23.8Hz,1H...
Embodiment 2
[0041] Example 2 Compound 2
[0042]
[0043] The procedure is the same as in Example 1, white solid (10mg, 35%). 1 H NMR(400MHz, DMSO-d 6 )δ7.24(s,5H), 5.37(dd,J=11.9,7.8Hz,2H), 4.89–4.75(m,1H), 4.68(s,1H), 4.52–4.39(m,1H), 4.34 --4.22(m,1H),4.05(s,1H),3.72--3.54(m,2H),3.23(s,3H),3.07(s,2H),2.95--2.73(m,2H),2.56(s ,4H), 2.45(dd,J=31.6,12.4Hz,2H),2.31–2.16(m,2H),2.06(dd,J=15.2,7.2Hz,6H),1.80–1.66(m,2H), 1.54–1.46(m,3H),1.12(d,J=4.4Hz,4H),1.01–0.87(m,13H).HRMS(ESI; m / z)[M+Na] + calcd for C 35 H 57 N 5 O 7 Na 694.4156, found 694.4106.
Embodiment 3
[0044] Example 3 Compound 3
[0045]
[0046] The procedure is the same as in Example 1, white solid (13 mg, 41%). 1 H NMR(400MHz, DMSO-d 6 )δ7.41–7.11(m,5H), 4.54(ddd,J=28.1,14.9,8.3Hz,1H), 4.32–4.20(m,1H), 4.19–4.09(m,1H), 4.08–4.00( m, 1H), 3.66 (dd, J = 12.2, 6.0 Hz, 1H), 3.48 (t, J = 7.7 Hz, 1H), 3.29 (t, J = 5.1 Hz, 3H), 3.20-3.14 (m, 2H ), 3.13 (s, 2H), 2.92 (dd, J = 12.2, 6.1 Hz, 1H), 2.87–2.77 (m, 1H), 2.33 (ddd, J = 17.8, 11.9, 5.6 Hz, 1H), 2.21– 2.06(m,2H),2.05-1.84(m,4H),1.68(dt,J=15.0,12.8Hz,2H),1.53-1.39(m,2H),1.34(s,2H),1.26(d, J=22.0Hz,8H),1.08–0.75(m,17H).HRMS(ESI;m / z)[M+H] + calcd for C 37 H 60 N 5 O 7 686.4493,found 686.4545.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com