Application of trichosanthin in sensitization and/or activation of dendritic cells
A technology of dendritic cells and trichosanthin, applied in the field of biomedicine, can solve problems that are difficult to prevent the occurrence and development of tumors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
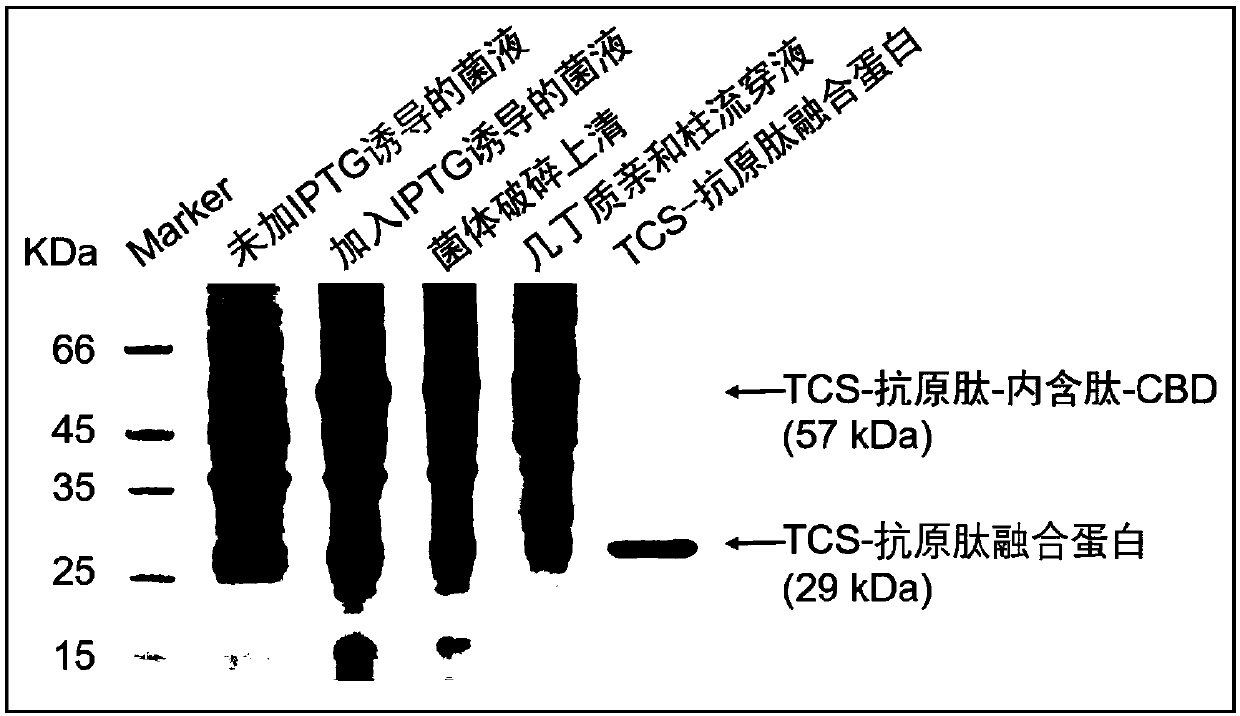
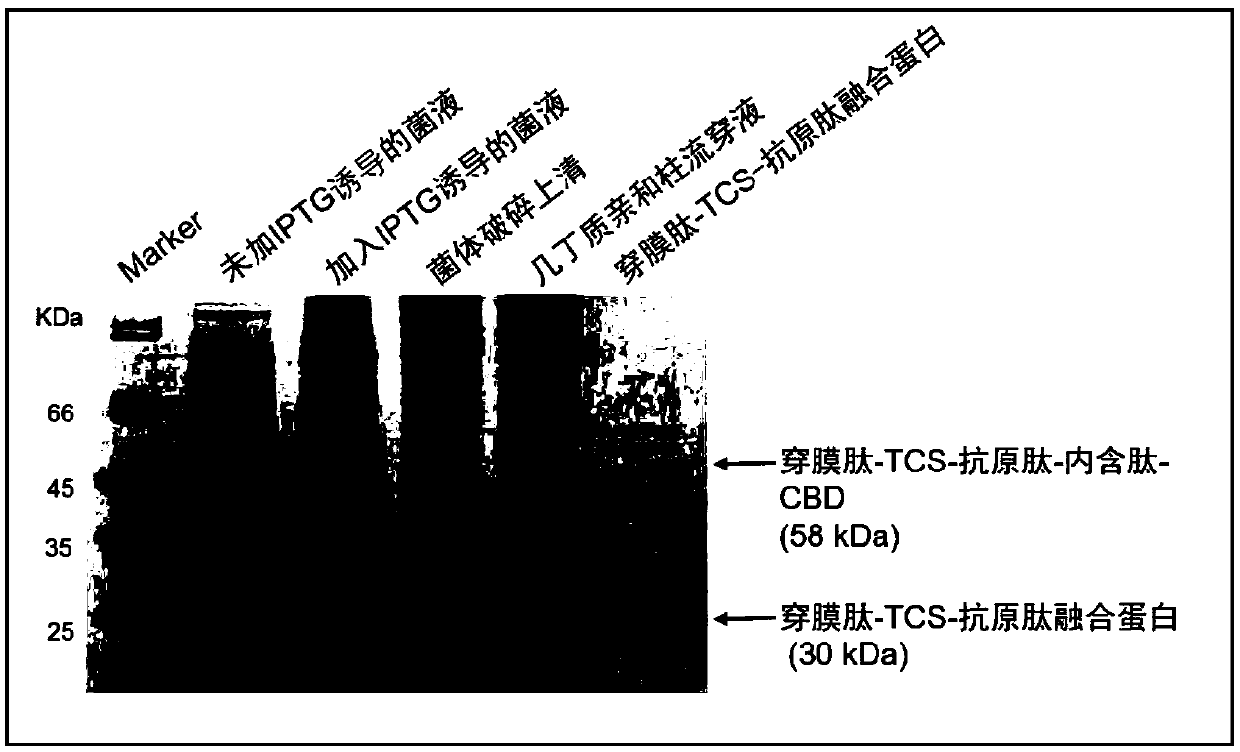
[0204] Preparation of fusion protein 1 (TCS-antigen peptide protein)
[0205] Prokaryotic expression and purification of TCS-antigen peptide protein
[0206] a: The constructed plasmid TCS-antigen peptide was transformed into Escherichia coli BL21(DE3) competent cells.
[0207]b: Transfer the strain containing the recombinant plasmid TCS-antigen peptide to LB medium containing 100 μg / ml Amp, and culture it in a constant temperature shaker at 37°C at 220rpm until the logarithmic growth phase (absorbance value at 600nm is 0.6-0.8), add IPTG with a final concentration of 1 mM was expressed overnight (16 h) at 25° C. and 150 rpm.
[0208] c: Use a centrifuge at 4°C and 9,000 rpm for 3 minutes to collect bacteria.
[0209] d: Resuspend the bacteria in HEPES buffer (containing 20 mM HEPES, 150 mM NaCl, 1 mM EDTA, 0.5‰ Tween 20, pH 8.5).
[0210] e: Use a probe sonicator with a power of 400 W to sonicate the cells for 35 minutes.
[0211] f: 12,000rpm, centrifuge at 4°C for 30min...
preparation Embodiment 2
[0217] Preparation of fusion protein 2 (penetrating peptide-TCS-antigen peptide protein)
[0218] Prokaryotic expression and purification of penetrating peptide-TCS-antigen peptide protein
[0219] a: The constructed plasmid-penetrating peptide-TCS-antigen peptide was transformed into Escherichia coli BL21(DE3) competent cells.
[0220] b: The strain containing the recombinant plasmid penetrating peptide-TCS-antigen peptide was transferred to the LB medium containing 100 μg / ml Amp, and cultured in a constant temperature shaker at 37°C at 220rpm to the logarithmic growth phase (absorbance value at 600nm was 0.6- 0.8), adding IPTG with a final concentration of 1 mM, and expressed overnight (16 h) at 37° C. and 220 rpm.
[0221] c: Use a pre-cooled centrifuge at 4°C and 9,000 rpm for 3 minutes to collect the bacteria.
[0222] d: Resuspend the bacteria in HEPES buffer (containing 20 mM HEPES, 150 mM NaCl, 1 mM EDTA, 0.5‰ Tween 20, pH 8.5).
[0223] e: The cells were sonicated ...
experiment Embodiment 1
[0230] MTT (3-(4,5-dimethylthiazole-2)-2,5-diphenyl bromotetrazolium blue, trade name: thiazolium blue) method for the determination of recombinant protein drugs TCS, TCS-antigenic peptide, Cytotoxic effect of penetrating peptide-TCS-antigen peptide Digest and count murine dendritic cells DC2.4 cells in the logarithmic growth phase, and dilute to a density of 4×10 4 The cell suspension of 1 cell / mL was transferred to a 96 cell culture well plate, and 100 μL of cell suspension was added to each well, and cultured with DMEM complete medium containing 10% calf serum for 12 h (37 °C, 5% CO 2 ).
[0231] Determine the optimal drug concentration range through preliminary experiments, add solutions with different concentrations, and make 6 replicate holes for each concentration. After culturing for 48 hours, 20 μl of MTT (5 mg / ml, purchased from Sigma-Aldrich, USA) was added and incubated for 4 hours. The culture supernatant was carefully aspirated, 200 μL of DMSO was added to each ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com