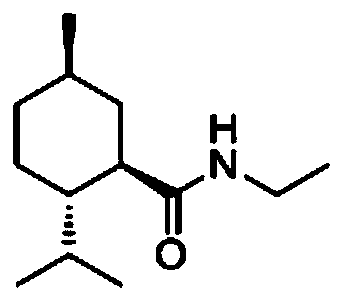
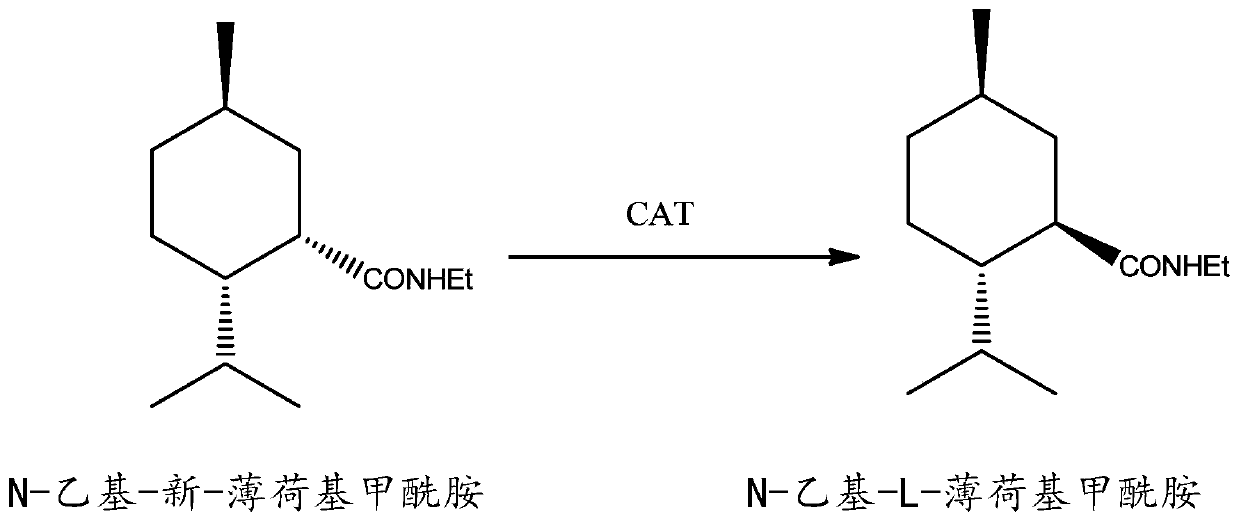
Method for preparing N-ethyl-L-menthyl formamide by configuration inversion of N-ethyl-neo-menthyl formamide
A technology of menthyl formamide and configuration inversion, applied in the field of configuration inversion of fine chemicals, can solve the problem of increasing equipment investment and production cost, increasing separation difficulty, and increasing the total yield of N-ethyl-L-menthyl formamide. The problem of unsatisfactory rate and other problems can achieve the effect of convenient post-reaction treatment, lower energy consumption and lower production cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] N-ethyl-neo-menthyl formamide and N-ethyl-L-menthyl formamide mixture 30g (N-ethyl-neo-menthyl formamide / N-ethyl-L-menthyl formamide= 0.38) was dissolved in 150ml of toluene solution, and 0.6g of N,N'-dicyclohexylmethylamine was added to the system. Stir at 135° C. for 24 h under nitrogen protection. After cooling down, add pure water to the reaction system (wash until the pH is between 7 and 7.5), let it stand and wait for stratification. Take out the organic layer and reclaim solvent toluene by distillation under reduced pressure, thereby obtain about 27.4g product, GC internal standard quantity: N-ethyl-neo-menthyl formamide / N-ethyl-L-menthyl formamide= 0.02, the conversion rate is 91.3%, and the selectivity is 99.3%.
Embodiment 2
[0029] N-ethyl-neo-menthyl formamide and N-ethyl-L-menthyl formamide mixture 30g (N-ethyl-neo-menthyl formamide / N-ethyl-L-menthyl formamide= 0.26) was dissolved in 150ml of toluene solution, and 0.6g of N,N'-dicyclohexylethylamine was added to the system. Stir at 135° C. for 24 h under nitrogen protection. After cooling down, add appropriate pure water to the reaction system to wash it until the pH is between 7 and 7.5, and let it stand for stratification. Toluene was reclaimed by distillation under reduced pressure, so as to obtain about 28.5g product, GC internal standard quantity: N-ethyl-neo-menthyl formamide / N-ethyl-L-menthyl formamide=0.01, conversion rate 95%, the selectivity is 99.1%.
Embodiment 3
[0031] N-ethyl-neo-menthyl formamide and N-ethyl-L-menthyl formamide mixture 50g (N-ethyl-neo-menthyl formamide / N-ethyl-L-menthyl formamide= 0.38) was dissolved in 250ml of toluene solution, and 2.5g of N,N'-diisopropylethylamine was added to the system. Stir at 135° C. for 24 h under nitrogen protection. Filtrate, add pure water to the filtrate and wash it until the pH is between 7 and 7.5, let it stand and wait for stratification, take out the organic layer after stratification and wash it twice with an appropriate amount of pure water, let it stand for stratification again, take out the organic layer under reduced pressure conditions Lower distillation reclaims toluene, thereby obtains about 46.7g product, GC internal standard quantity: N-ethyl-neo-menthyl formamide / N-ethyl-L-menthyl formamide=0.025, conversion rate 93%, select The sex is 98.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com