Application of regulatory peptide and mansonone E in preparation of improved medicament for treating leukemia and application of regulatory peptide and mansonone E
A technology for leukemia and mansonone, which is applied in the field of biopharmaceuticals to achieve the effects of moderate cost, convenient medication and small side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1 The cultivation of K562 leukemia cells
[0034]Tumor cell line: K562 leukemia cells (preserved by Hematology Laboratory of Shaanxi Provincial People's Hospital). After recovering from liquid nitrogen, K562 cells were placed in RPMI-1640 culture medium containing 10% inactivated fresh calf serum at 37°C. The volume fraction was 5% CO2, cultured under saturated humidity conditions, and the cell viability was kept >97%. The K562 cells (1X 105 / ml) in the exponential growth phase were divided into the control group and the experimental group for future use.
Embodiment 2
[0035] Example 2 Cell Proliferation Inhibition Experiment
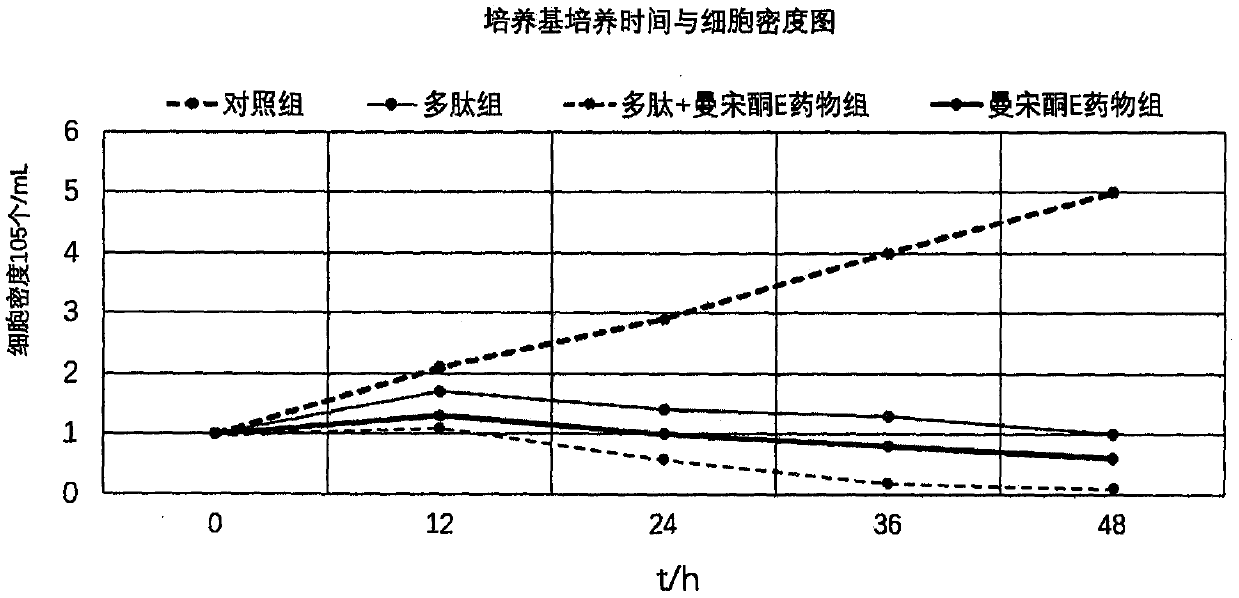
[0036] Cell proliferation inhibition experiment: Add K562 cells in the logarithmic growth phase to 24-well plate at 1×105 / mL, 1 mL per well, the drugs were 10 μg / mL polypeptide group, 25 μg / mL mansone E drug group, 10 μg / mL polypeptide + 25 μg / mL manthonone E drug group, PBS blank control group, counted after 12, 24, 36, and 48 hours, counted live cells by trypan blue exclusion test, repeated the experiment 3 times, and took mean, to plot growth curves, see figure 1 Shown, wherein the polypeptide sequence is shown in SEQ ID NO: 1.
[0037] from figure 1 It can be seen that the inhibitory effect of peptides alone on cells is very weak, while the combination of peptides and mansonone E can significantly inhibit cell proliferation and promote cell apoptosis, and the cell survival rate is less than 5% at 48 hours. Exhibited an excellent inhibitory effect.
Embodiment 3
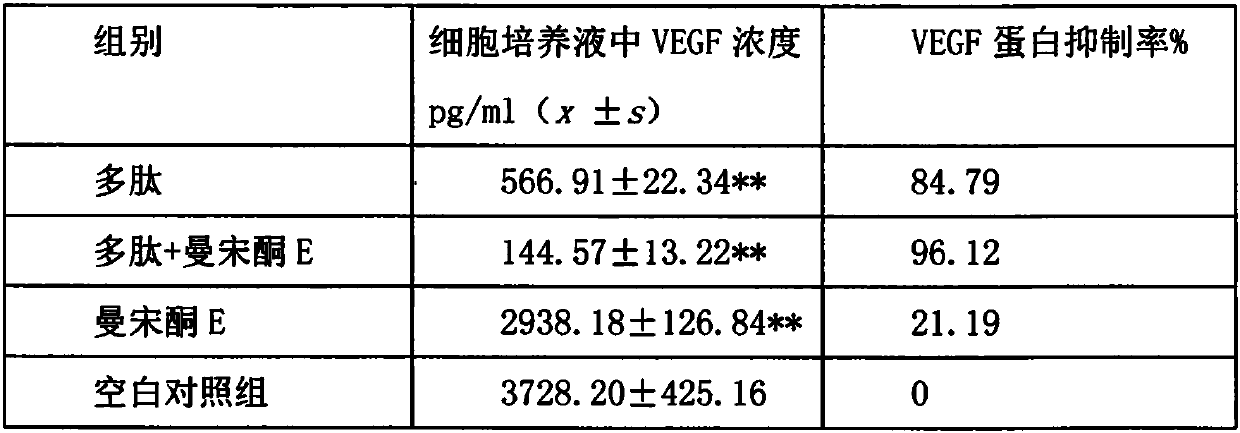
[0038] The expression measurement of embodiment 3 protein
[0039] Determination of protein expression by Western blotting: Take K562 cells in the logarithmic growth phase, centrifuge, prepare a cell suspension with a concentration of 3×105 / mL in RPMI 1640 medium containing 10% fetal bovine serum, and place in a 6-well plate Inoculate 1 mL per well, place the plate at 37°C, 5% CO 2 incubator. Add 10 μg / mL polypeptide group, 25 μg / mL mansonone E drug group, 10 μg / mL polypeptide+25 μg / mL mansonone E drug group, PBS blank control group, centrifuge after 0 and 48 hours after administration respectively Collect cells, lyse cells with protein lysate, centrifuge to remove cell debris, cook at 95°C for 10 min, quantify protein, 40 μg / well, and load on 10% SDS-polypropylene gel electrophoresis. After the protein on the gel was transferred to the PVDF membrane, it was blocked overnight in 5% skimmed milk, and the primary antibody was added according to the antibody dilution ratio to i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com