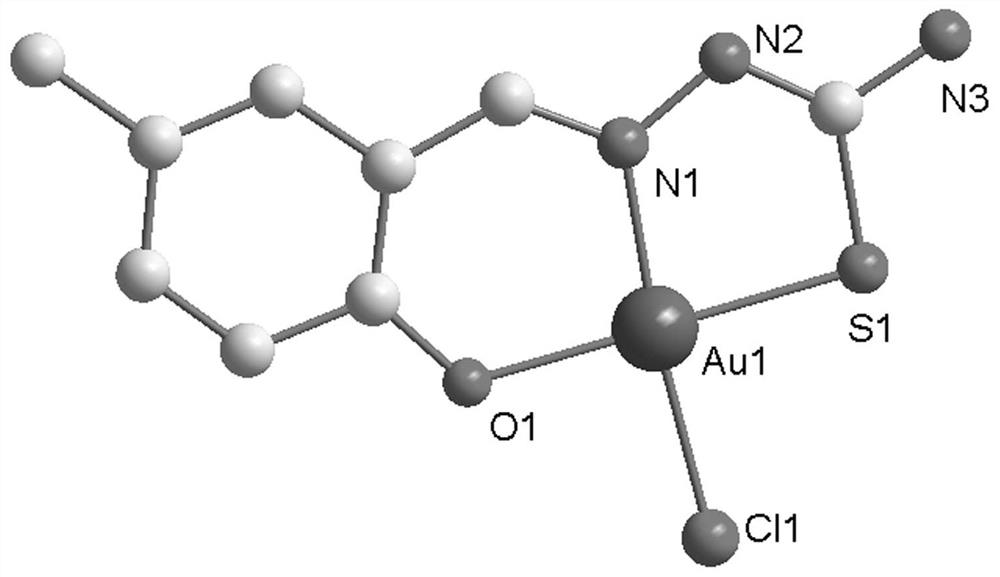
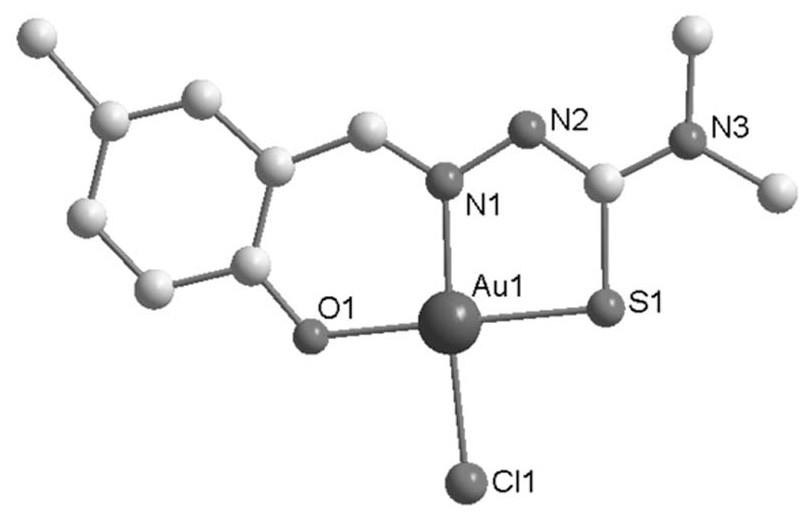
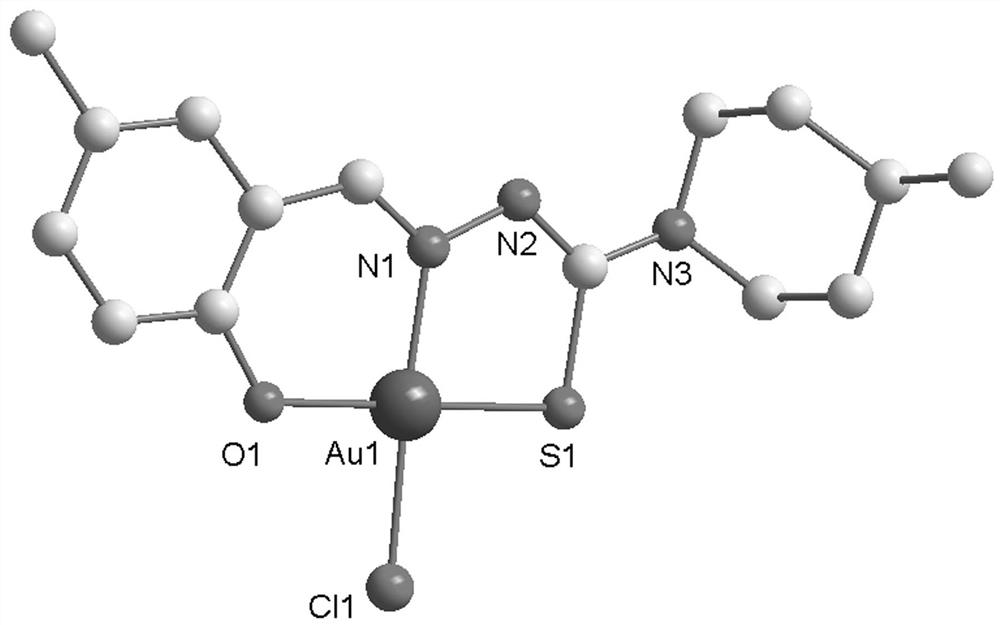
Synthesis method and application of gold (iii) metal complex with human serum albumin as carrier
A technology of human serum albumin and metal complexes, applied in the direction of gold organic compounds, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve tumor tissue accumulation, limit anti-tumor applications, and metal complexes issues such as lack of targeting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The synthetic method of C1 metal complex is:
[0029] 1) Dissolve 408mg (3mmol) of 5-methylsalicylaldehyde in 20ml of methanol. After dissolving, add 273mg (3mmol) of 3-thiosemicarbazide and mix well to obtain a mixed solution. Reflux the mixed solution at 65°C for 4h, filter, and The cake was washed 2-3 times with methanol to obtain ligand 1;
[0030] Yield: 0.62g, 98.8%, white solid; R f =0.314(Petroleum ether:EtOAc=2:1).m.p.123-125℃. 1 H NMR (400MHz, DMSO-d 6 )δ11.34(s,1H,OH),9.60(s,1H,NH),8.33(s,1H,NCH),8.10,7.90(2s,2H,NH 2 ),7.74(s,1H),7.01(dd,J=8.4,2.3Hz,1H),6.75(d,J=8.3Hz,1H),2.20(s,3H). 13 C NMR (100MHz, DMSO-d 6 )δ177.59, 154.25, 139.74, 131.70, 127.80, 126.67, 119.87, 115.89, 20.00.ESI + m / z: calcd for C 9 h 10 N 3 OS, 208 [M-H] - .
[0031] 2) After dissolving 20.1 mg (0.1 mmol) of Ligand 1 prepared in step 1) in 10 ml of methanol, add 39.7 mg (0.1 mmol) of Na[AuCl 4 ]·2H 2 O, stirred at room temperature for 12 hours, filtered to obtain a yellow-...
Embodiment 2
[0034] The synthetic method of C2 metal complex is:
[0035] 1) Dissolve 408mg (3mmol) of 5-methyl salicylaldehyde in 20ml of methanol. After dissolving, add 357mg (3mmol) of 4,4-dimethyl-thiosemicarbazide and mix well to obtain a mixed solution. Put the mixed solution at 65°C Reflux for 4 hours, filter, and wash the filter cake 2-3 times with methanol to obtain Ligand 2;
[0036] Yield: 0.59g, 82.98%, white solid; R f =0.414(Petroleum ether:EtOAc=2:1).m.p.128-130℃. 1 H NMR (400MHz, DMSO-d 6 )δ11.49(s,1H,OH),11.21(s,1H,NH),8.45(s,1H,NCH),7.16(d,J=2.1Hz,1H),7.07(dd,J=8.2, 2.2Hz, 1H), 6.79(d, J=8.3Hz, 1H), 3.29(s, 6H), 2.24(s, 3H). 13 C NMR (100MHz, DMSO-d 6 )δ179.19, 154.91, 146.31, 131.33, 129.99, 127.46, 118.01, 116.32, 40.97, 19.87.C 11 h 14 N 3 OS 236[M-H] - .
[0037]2) After dissolving 23.7mg (0.1mmol) of ligand 2 prepared in step 1) in 10ml of methanol, add 39.7mg (0.1mmol) of Na[AuCl 4 ]·2H 2 O, stirred at room temperature for 12 hours, filtered to obtain a ...
Embodiment 3
[0040] The synthetic method of C3 metal complex is:
[0041] 1) 408mg (3mmol) 5-methyl salicylaldehyde was dissolved in 20ml methanol, after dissolving, 519mg (3mmol) 3-(4-methylpiperidine)-thiosemicarbazide was added and mixed uniformly to obtain a mixed solution, which was mixed in Reflux at 65°C for 4 hours, filter, and wash the filter cake with methanol 2-3 times to obtain Ligand 3;
[0042] Yield: 0.71g, 81.32%, white solid; R f =0.411 (Petroleum ether:EtOAc=2:1).m.p.134-136°C. 1 H NMR (400MHz, DMSO-d 6 )δ11.39(s,1H,OH),11.35(s,1H,NH),8.40(s,1H,NCH),7.17(d,J=1.8Hz,1H),7.06(dd,J=8.3, 2.0Hz, 1H), 6.78(d, J=8.3Hz, 1H), 4.69(d, J=13.1Hz, 2H), 3.12-3.06(m, 2H), 2.24(s, 3H), 1.73-1.65( m,3H),1.19-1.05(m,2H),0.92(d,J=6.1Hz,3H). 13 C NMR (100MHz, DMSO-d 6 )δ178.60, 154.86, 145.86, 131.32, 129.80, 127.46, 118.13, 116.29, 48.78, 33.58, 30.20, 21.33, 19.88.ESI + m / z: calcd for C 15 h 20 N 3 OS, 290 [M-H] - .
[0043] 2) After dissolving 29.1 mg (0.1 mmol) of ligand 3 pre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com