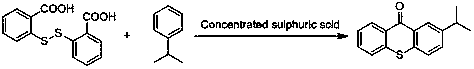
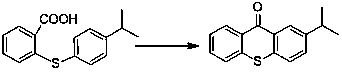
Preparation method of 2-isopropylthioxanthone
A technology of isopropylthioxanthone and propylphenylthio group is applied in the field of preparation of photoinitiator 2-isopropylthioxanthone, which can solve the problem of high sewage treatment pressure, large amount of concentrated sulfuric acid, and poor product appearance. It can achieve the effect of less environmental pressure, less dosage and cost saving.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: Preparation of 2-(4-cumylthio)benzoic acid
[0033] Heat 120.3g (0.79mol) of o-chlorobenzoic acid, 120g (0.79mol) of 4-isopropylthiophenol, and 68.0g (1.70mol) of sodium hydroxide to 80°C and stir for 0.5 hours, add 520mL of toluene, and heat Reflux for dehydration, after removing 14mL of water. Protect the obtained solution with nitrogen, seal and pressurize at 1 MPa, heat to 210°C, react for 10 hours, cool down, recover toluene from the solvent, adjust the pH of the solution to 2 with dilute hydrochloric acid or dilute sulfuric acid, filter with suction and dry to prepare 204 g of 2-(4-isopropylphenylthio)benzoic acid, melting point 149-151°C.
Embodiment 2
[0034] Embodiment 2: Preparation of 2-isopropylthioxanthone
[0035] Take 136.2g (0.50mol) of 2-(4-isopropylphenylsulfanyl)benzoic acid and dissolve it in 250mL of dichloroethane, and slowly add 125.0g of 98% sulfuric acid (1.25mol) dropwise under a water bath for 30 minutes, slowly Heat up, reflux for dehydration, monitor the reaction according to the amount of separated water by TLC or liquid phase, after the reaction is complete, lower the temperature and add 150mL of water, stir for 30min, let stand for stratification, separate the organic phase, and the acidic water phase for later use. The organic phase was washed with 150 mL of water, and then the solvent was recovered by distillation. The residue was recrystallized with 250 mL of absolute ethanol to obtain 114.5 g of a light yellow solid, namely: 2-isopropylthioxanthone with a content of 99.4%.
Embodiment 3
[0036] Embodiment 3: Preparation of 2-isopropylthioxanthone
[0037] Take 136.2g (0.50mol) of 2-(4-isopropylphenylsulfanyl)benzoic acid and disperse it in 400mL of cyclohexane, slowly add 125.0g of 98% sulfuric acid (1.25mol) dropwise in a water bath, after 30min, slowly heat Heat up, reflux and dehydrate, monitor the reaction according to the amount of separated water by TLC or liquid phase, after the reaction is complete, cool down and add 150mL of water, stir for 30min, let stand for stratification, separate the organic phase, then wash with 150mL of water, and then distill to recover the solvent. The material was recrystallized with 250 mL of absolute ethanol to obtain 115.8 g of a light yellow solid, namely: 2-isopropylthioxanthone, with a content of 99.3%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com