Fluorohydroxyproline derivatives useful in the preparation of proteolysis targeted chimeras
一种化合物、水合物的技术,应用在合成新型PROTACs中的应用领域,能够解决有害的副作用毒性、增强脱靶效应等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0236] Preparation process of 3-fluorohydroxyproline (F-HYP)
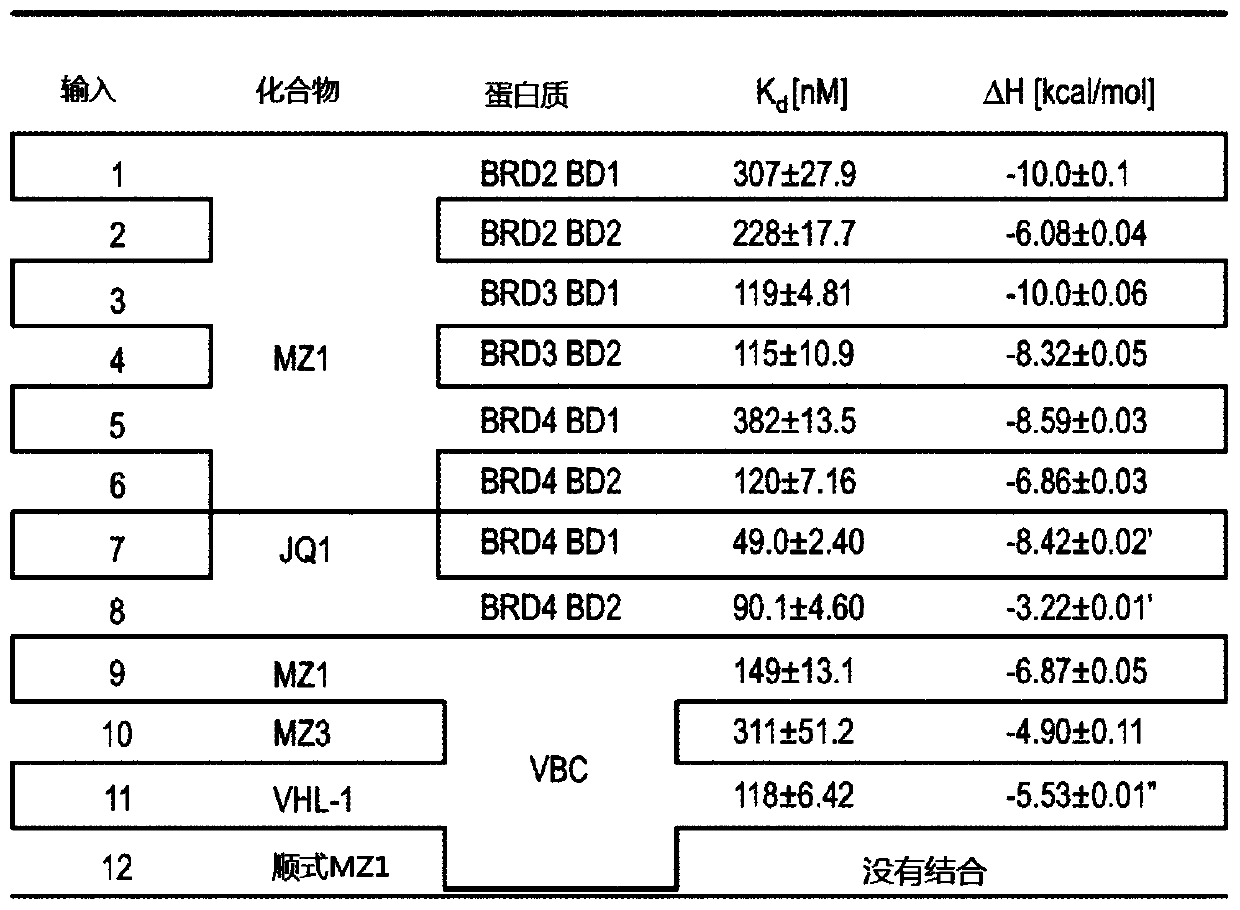
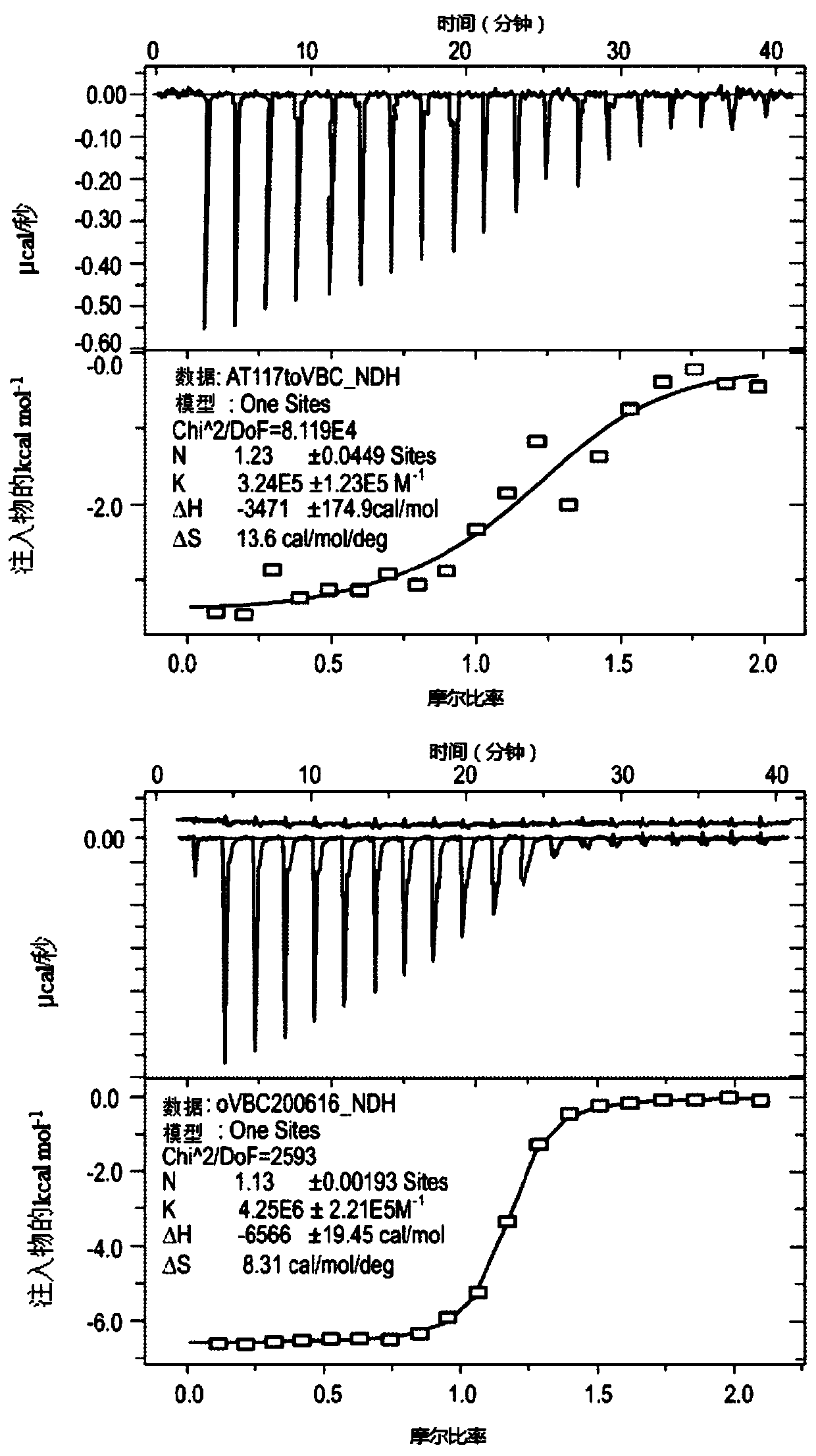
[0237] As indicated above, applicants have developed a novel process for the preparation of 3-fluoro-hydroxyproline intermediate compounds for the preparation of compounds of formula A. Compounds of formula A comprising, for example, a 3-fluoro-hydroxyproline central scaffold are useful for providing novel VHL binding ligand compounds of structures A-L comprising a 3-fluoro-hydroxyproline central scaffold. The general procedure for providing two such 3-fluoro-hydroxyprolines, novel VHL conjugates with a central scaffold of 3-fluoro-hydroxyproline, and PROTACs of structure A-L-B, wherein formula A The compounds are based on the 3-fluoro-hydroxyproline central scaffold.
[0238] Fluoroprolines are fluorinated amino acids that are readily obtained from the interconversion of the hydroxyproline functional group. (2S,4R)-4-hydroxyproline, or L-hydroxyproline (C 5 h 9 o 3 N), with the chemical name 4-hydroxypyrrolid...
Embodiment
[0304] Example compound 18e: (2R, 3S, 4S)-1-((S)-1-(4-((2S, 4R)-1-acetyl-4-(4-chlorobenzyl)-2-methyl Base-1,2,3,4-tetrahydroquinolin-6-yl)phenyl)-12-(tert-butyl)-1,10-dioxo-5,8-dioxa-2,11- Diazatridecane-13-yl)-3-fluoro-4-hydroxy-N-(4-(4-methylthiazol-5-yl)benzyl)pyrrolidine-2-carboxamide;
[0305] or a pharmaceutically acceptable salt, enantiomer, stereoisomer, hydrate, solvate, or polycrystal thereof.
[0306] As discussed above, there is provided a PROTAC compound having the structure A-L-B, wherein A is an E3 ubiquitin ligase protein-binding ligand compound of formula I, preferably of formula IA, wherein L and B are any of the above detailed descriptions. In one aspect, wherein the L group is in the R 1 , R 3 , R 4 , R 5 or R 8 The position is directly bound to the compound of formula I or IA. L group in R 1 , R 3 , R 4 , R 5 or R 8 Exemplary compounds at positions that bind directly to compounds of Formula I or IA are provided in Groups I to V below.
[0307]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com