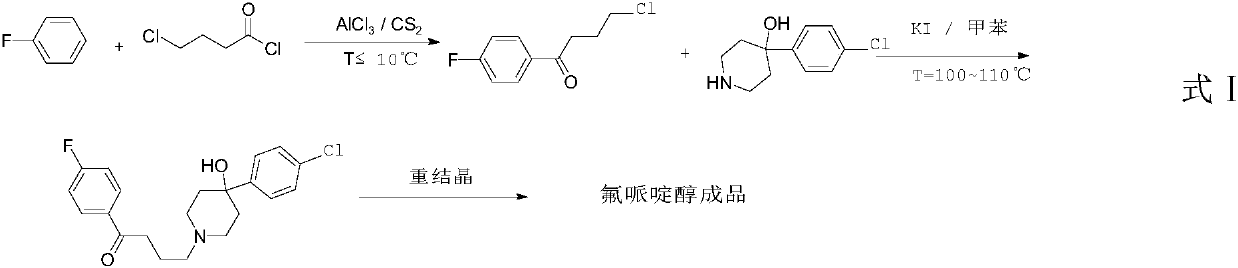
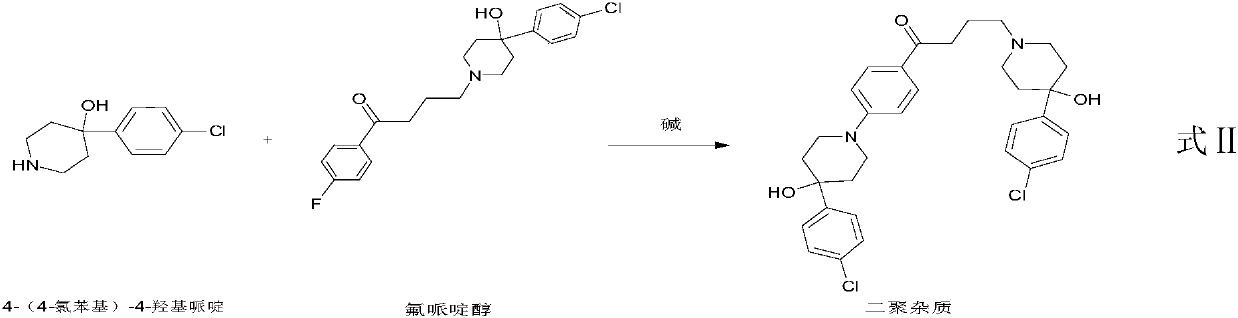
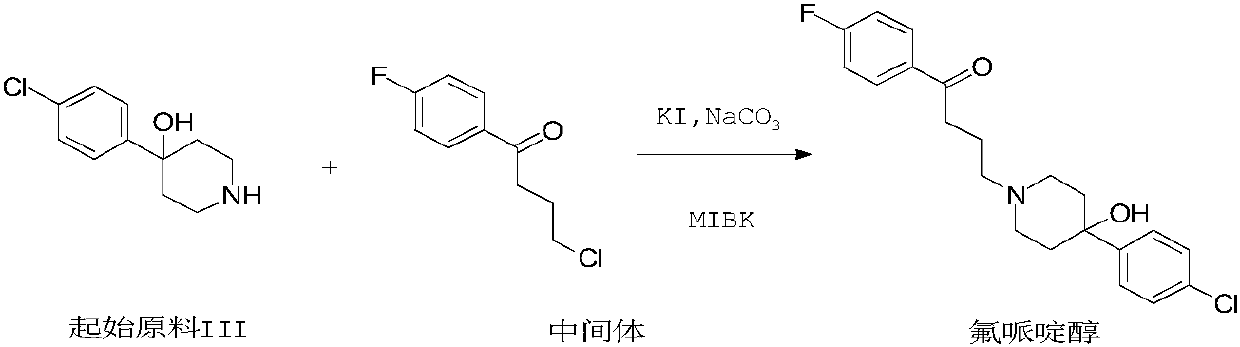
Purification method of haloperidol
A technology of haloperidol and a purification method, applied in the direction of organic chemistry, which can solve the problems of difficult removal of dimerization impurities and inability to obtain active drug molecules with qualified purity, and achieve the effect of high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1 Preparation of crude haloperidol (170826 batches, dimerization impurity content: 0.38w / w%)
[0021]
[0022] Add starting material III (10.01g), intermediate (28.40g), sodium carbonate (10.01g), potassium iodide (3.92g) and methyl isobutyl ketone (200mL) into a 500mL reaction flask, heat up to 130°C, heat and stir , reacted for 6 hours (HPLC detection of starting material III<5.0%), cooled to 50°C, added water (100mL) and stirred for 0.5h, separated the organic layer, and the organic layer continued to drop to 10-20°C for crystallization, filtered, and used for filter cake A small amount of methyl isobutyl ketone was washed, and air-dried at 55°C to obtain 14.7 g of the product, with a yield of 82.7%.
Embodiment 2
[0023] Example 2 Preparation of crude haloperidol (170808 batches, dimerization impurity content: 0.57w / w%)
[0024] Add starting material III (20.1g), intermediate (57.0g), potassium iodide (0.80g), sodium carbonate (20.0g) and methyl isobutyl ketone (600mL) into the reaction flask, heat up and stir, and react at 130°C for 6 hours (HPLC detection starting material III<5.0%), cooled to 50°C, added water (200mL) and stirred for 0.5h, separated the organic layer, and the organic layer continued to drop to 10°C-20°C for crystallization, filtered, and the filter cake was washed with a small amount of formazan Washed with isobutyl ketone and air-dried at 55°C to obtain 30.0 g of the product with a yield of 84.1%.
Embodiment 3
[0025] The refining of embodiment 3 haloperidol crude product
[0026] Add 1.0g of the crude product (batch 170821-2) and 10mL of absolute ethanol to a 100mL reaction bottle, stir and heat to 70°C, after dissolving, add 2mL of water dropwise, the system is turbid, cool down to 10°C-20°C, filter, 55°C Air-dried to obtain 0.80 g, yield 80.0%.
[0027] In this example, the refining solvent: ethanol / water, the purity of haloperidol in the crude product is 89.37%, and 99.12% after purification, and the content of dimer impurities is about 0.4w / w%, indicating that the removal of dimer impurities is not significant, and other impurities Has been visibly removed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com