Amino acid chiral ligand containing bidentate coordination group, chiral catalyst, and corresponding preparation methods and applications thereof
A technology of chiral catalysts and coordination groups, applied in the field of asymmetric catalytic synthesis, can solve the problems of inability to effectively control central chirality and cannot be applied, and achieve enhanced chiral control capabilities, expanded diversity, and wide sources Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0053] The present invention will be further described below in combination with specific embodiments.
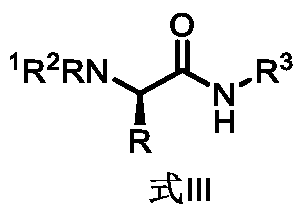
[0054] A chiral amino acid ligand containing a bidentate coordination group of the present invention has the following general structural formula:
[0055]
[0056] Wherein, R is selected from any one of methyl, ethyl, benzyl, phenyl, isopropyl or tert-butyl or tert-butyl derivative groups;
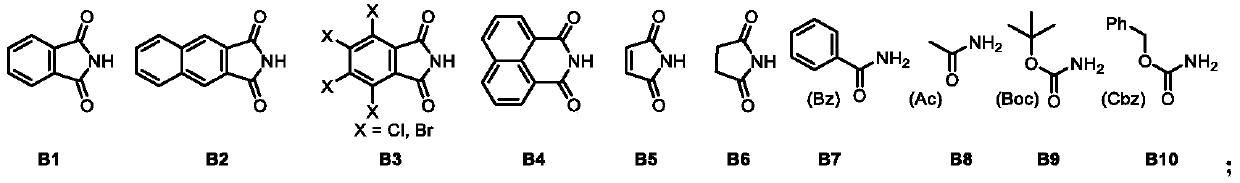
[0057] NR 1 R 2 Any one selected from the following groups;
[0058]
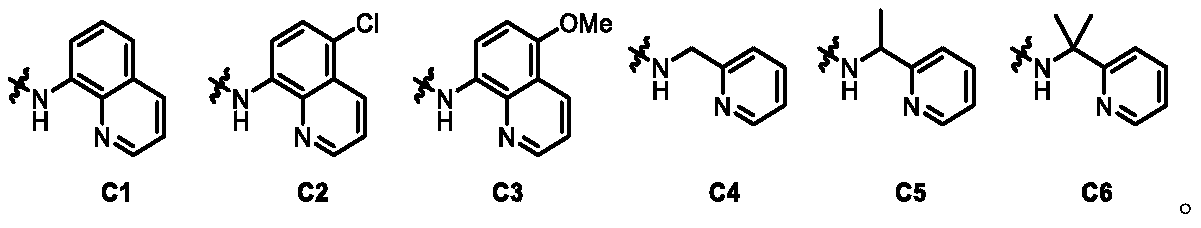
[0059] R 3 Any one selected from the following groups;
[0060]
[0061] Wherein, when R is selected from methyl, ethyl, benzyl, phenyl, isopropyl or tert-butyl, the corresponding amino acid chiral ligands containing bidentate coordination groups are uniformly prepared by the following steps:
[0062]
[0063] The first step: react the compound of formula I with acid anhydride under the reaction condition of a or the compound of formula I and acid chloride under the reaction condition of b to prepar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com