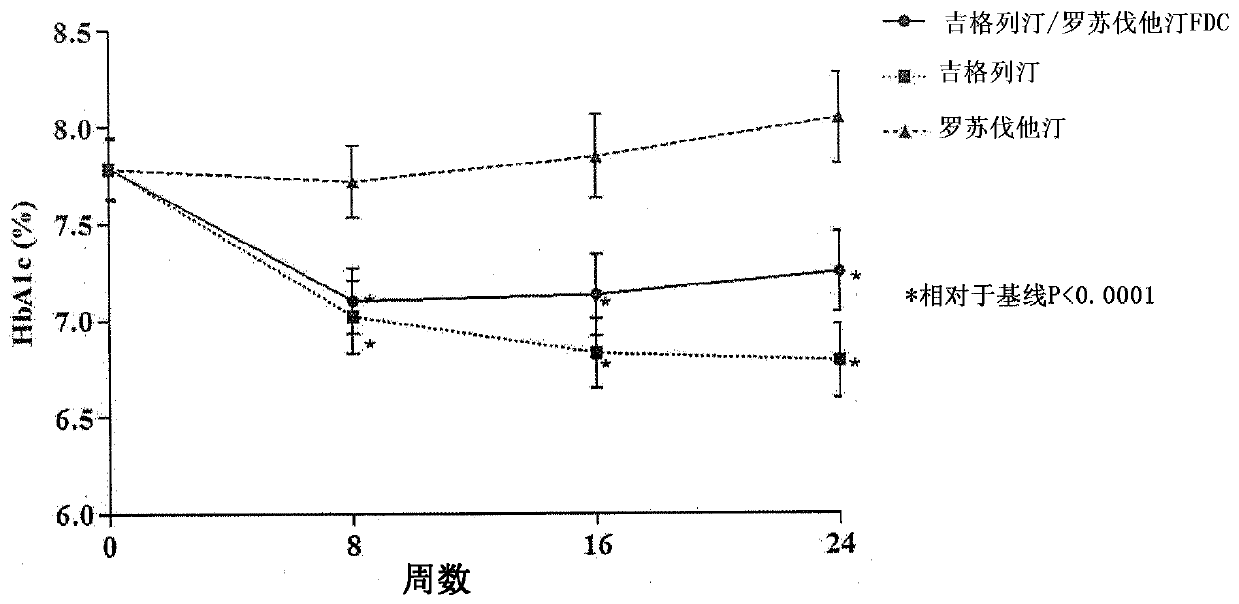
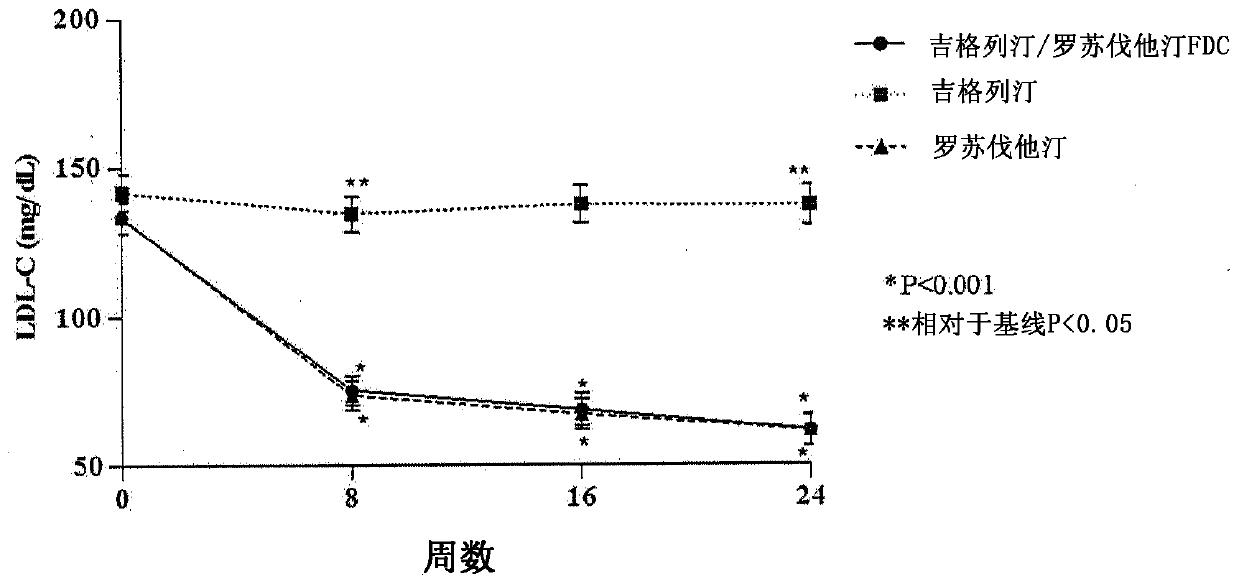
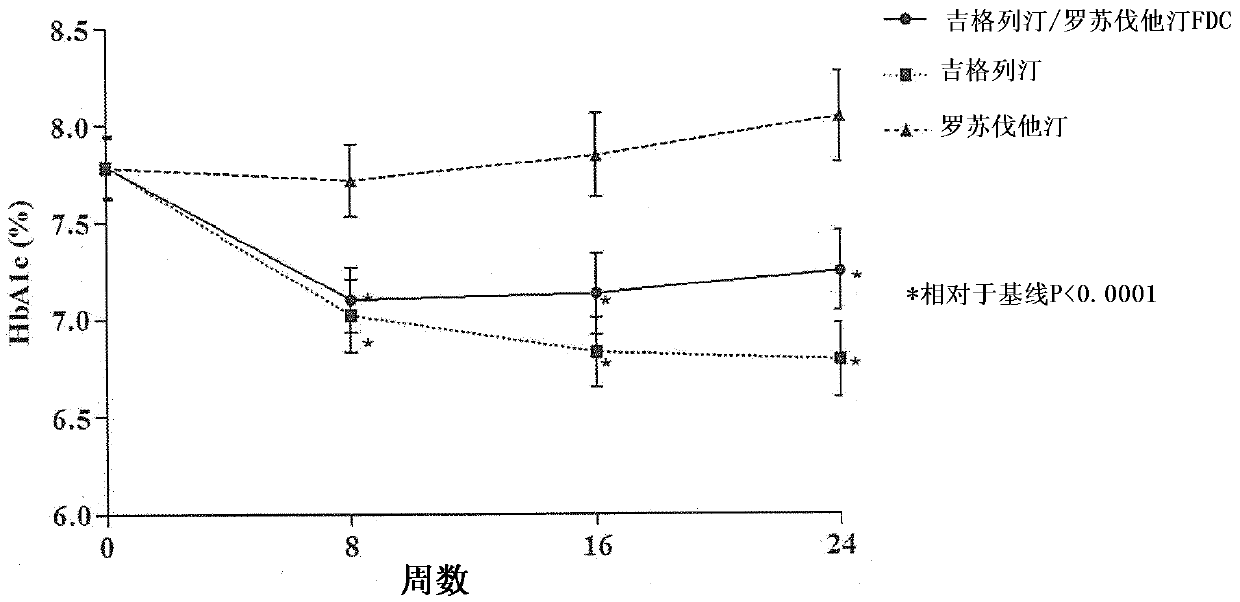
Medicinal complex for treating type 2 diabetes and diabetic dyslipidemia
A pharmacy and dosage form technology, applied in the field of combined preparations of type 2 diabetes and dyslipidemia, can solve problems such as increasing the risk of cardiovascular disease, and achieve the effect of improving drug compliance and treating dyslipidemia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: the preparation of fixed dosage
[0036] According to the composition of Table 1, fixed-dose combinations of 3 formulations were prepared, in which the content of rosuvastatin was different.
[0037] [Table 1]
[0038]
[0039] 1) Supertab 21AN: lactose anhydrous
[0040] 2) Prosolv SMCC 50: Silicified microcrystalline cellulose
[0041] 3) DI-TAB: Calcium hydrogen phosphate dihydrate
[0042] 4) Kollidon VA64: vinylpyrrolidone-vinyl acetate copolymer
Embodiment 2
[0043] Embodiment 2: the preparation of bilayer tablet
[0044] According to the composition of Table 2, two-layer tablets of 3 kinds of preparations were prepared, and in the 3 kinds of preparations, the content of rosuvastatin was different. The preparation method is presented in Table 3.
[0045] [Table 2]
[0046]
[0047]
[0048] 1) Flowlac 100: lactose monohydrate
[0049] 2) DI-TAB: Calcium hydrogen phosphate dihydrate
[0050] 3) Kollidon VA63: vinylpyrrolidone-vinyl acetate copolymer
[0051] [table 3]
[0052]
[0053]
Embodiment 3
[0054] Embodiment 3: stability test
[0055] The stability test of the bilayer tablet formulation prepared in Example 2 was carried out under accelerated conditions (40° C., 75% humidity) for 6 months. The results are presented in Table 4.
[0056] [Table 4]
[0057]
[0058] DP-IMP-1:
[0059] 2-[(2S)-6,6-Difluoro-2,3,5,6,7,8-hexahydroimidazo[1,2-a]pyridin-2-yl]-1-[2,4 -Bis(trifluoromethyl)-5,6,7,8-tetrahydropyrido[3,4-d]pyrimidin-7-yl]-1-ethanone
[0060] DP-IMP-2:
[0061] 2,4-bis(trifluoromethyl)-5,6,7,8-tetrahydropyrido[3,4-d]pyrimidin-8-one
[0062] DP-IMP-3:
[0063] (3S)-3-amino-4-(5,5-difluoro-2-oxopiperidinyl)-1-[8-hydroxy-2,4-bis(trifluoromethyl)-5,6, 7,8-Tetrahydropyrido[3,4-d]pyrimidin-7-yl]butan-1-one tartrate
[0064] 5-oxo:
[0065] Bis(6E)-7-[4-(4-fluorophenyl)-6-isopropyl-2-[methyl(methylsulfonyl)amino]pyrimidin-5-yl](3R)-3-hydroxy -5-oxohept-6-enoic acid
[0066] Lactone:
[0067] N-(4-(4-fluorophenyl)-5-(1E)-2-[(2S,4R)-4-hydroxy-6-oxotetrah...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com