Method for the preparation of polymers from monomers comprising laurolactam
A technology of cinnamyl lactam and polymer, which is applied in the field of polymers and can solve the problems of polyamide yellowing and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0031] The following examples describe the preparation of polyamide 12 according to various processes according to the invention and reference examples not according to the invention.
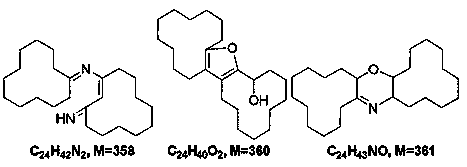
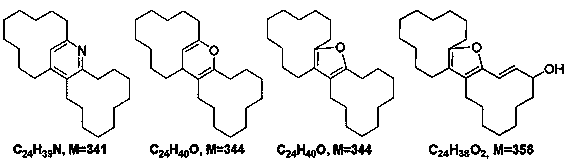
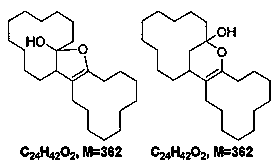
[0032] The term "impurity" used hereinafter refers to the following definition: polycyclic substances containing 24 carbon atoms and at least one heteroatom selected from oxygen and nitrogen, having a molar mass of 300 to 380 g / mol.
Embodiment A
[0033] Example A: Preparation of laurolactam
Embodiment A1
[0034] Example A1: with H 2 SO 4 Preparation of laurolactam and subsequent distillation
[0035]3.44 kg of a 25% by weight solution of cyclododecanone in hydrocumene were introduced into the 20-liter reactor at 90°C. Then 2.83 kg ammonia solution (25% by weight in water), 171 g ammonium sulfate, 22 g Hostapur® (emulsifier), 15.2 g titanium silicalite TS-1 and 1.84 kg water were added. The biphasic mixture was vigorously stirred, and 550 g H 2 o 2 The solution (70% by weight in water) was metered into the reactor. Finally, GC analysis showed complete conversion of cyclododecanone to cyclododecanone oxime (Cyclododecanoxim). Stirring was stopped to allow the phases to separate. The aqueous phase was drained and the organic phase was washed twice with 5 kg of water at 95°C.
[0036] Then 900 g of concentrated sulfuric acid were added to the solution of Cyclododecanoxim obtained in the last step in hydrocumene. The biphasic mixture was stirred at 50°C for 30 minutes. T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com