Application of panatinib in preparation of medicament for treating influenza virus infection
A technology of influenza virus infection and ponatinib, applied in the field of medicine, can solve problems such as undiscovered, and achieve the effect of reducing the degree of weight loss, reducing the time of clinical trials, and saving costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
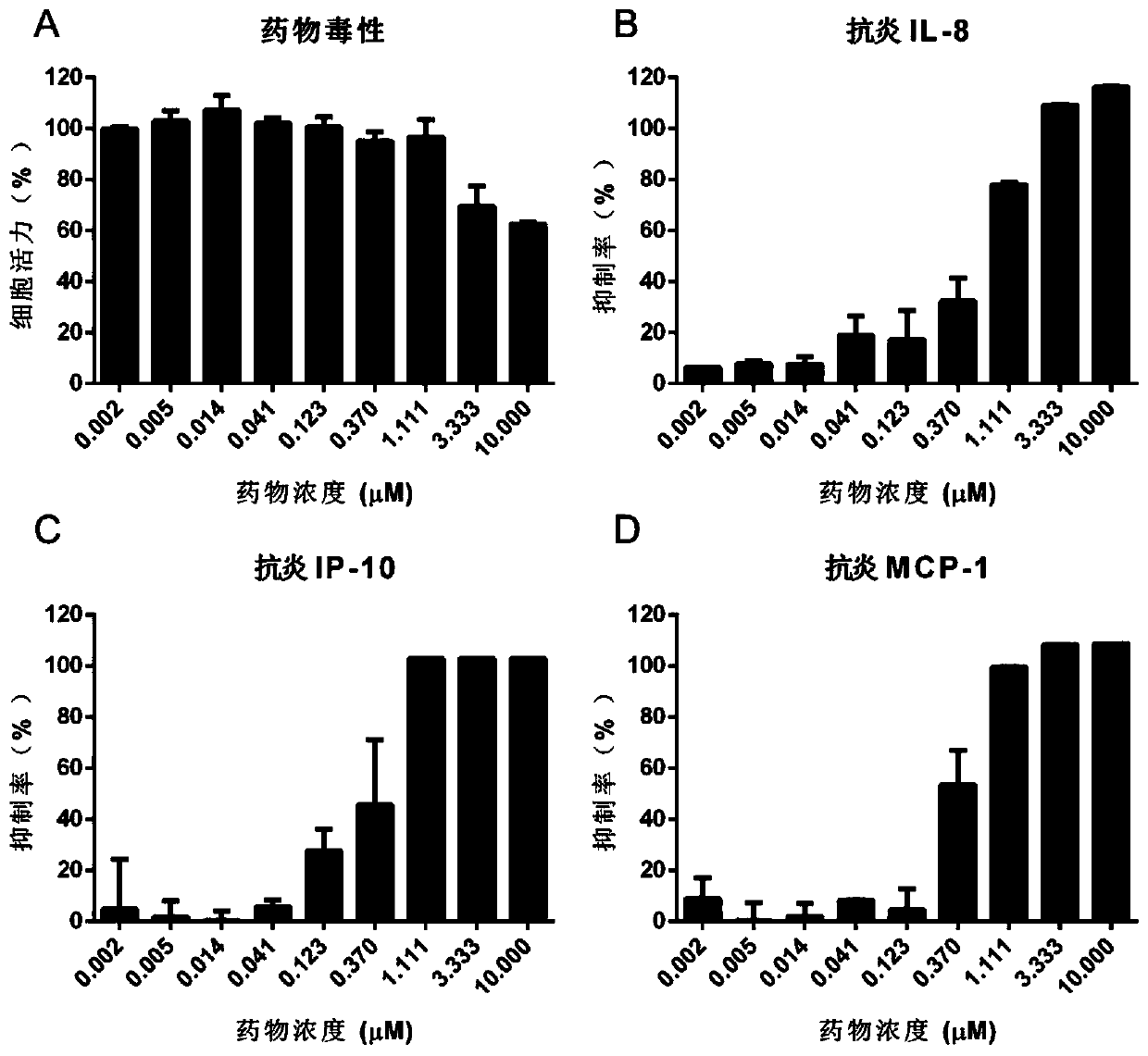
[0049] Example 1: Evaluation of anti-influenza virus-induced inflammatory activity of ponatinib in U937 cell line
[0050] 1 cell culture
[0051] After two subcultures, the frozen and revived cells were cultured in RPMI-1640 medium containing 10% fetal bovine serum and double antibodies (penicillin 100U / ml, streptomycin 100ug / ml), and the seeding density was not low. at 5x10 5 cell / ml, passage density not higher than 2x10 6 cell / ml.
[0052] 2 Cytotoxicity detection of ponatinib
[0053] U937 cells by 1.5×10 5 Cells / well (volume 100 μL) were seeded in a 96-well cell culture plate; the drug was prepared with 100 μL culture medium (RPMI-1640 medium + 10% serum + double antibody) per well, and added to the corresponding cell wells for mixing. Nine concentration gradients were set for the drug, and two replicate wells were set for each gradient concentration, and the final concentrations were 0.002 μM, 0.005 μM, 0.014 μM, 0.041 μM, 0.123 μM, 0.37 μM, 1.111 μM, 3.333 μM and 1...
Embodiment 2
[0065] Example 2: Evaluation of ponatinib's anti-inflammatory activity against other influenza viruses in U937 cell line
[0066] In this example, the U937 cell line was infected with the H3N2 subtype (A / Human / Hubei / 3 / 2005), and the specific method was the same as that in Example 1.
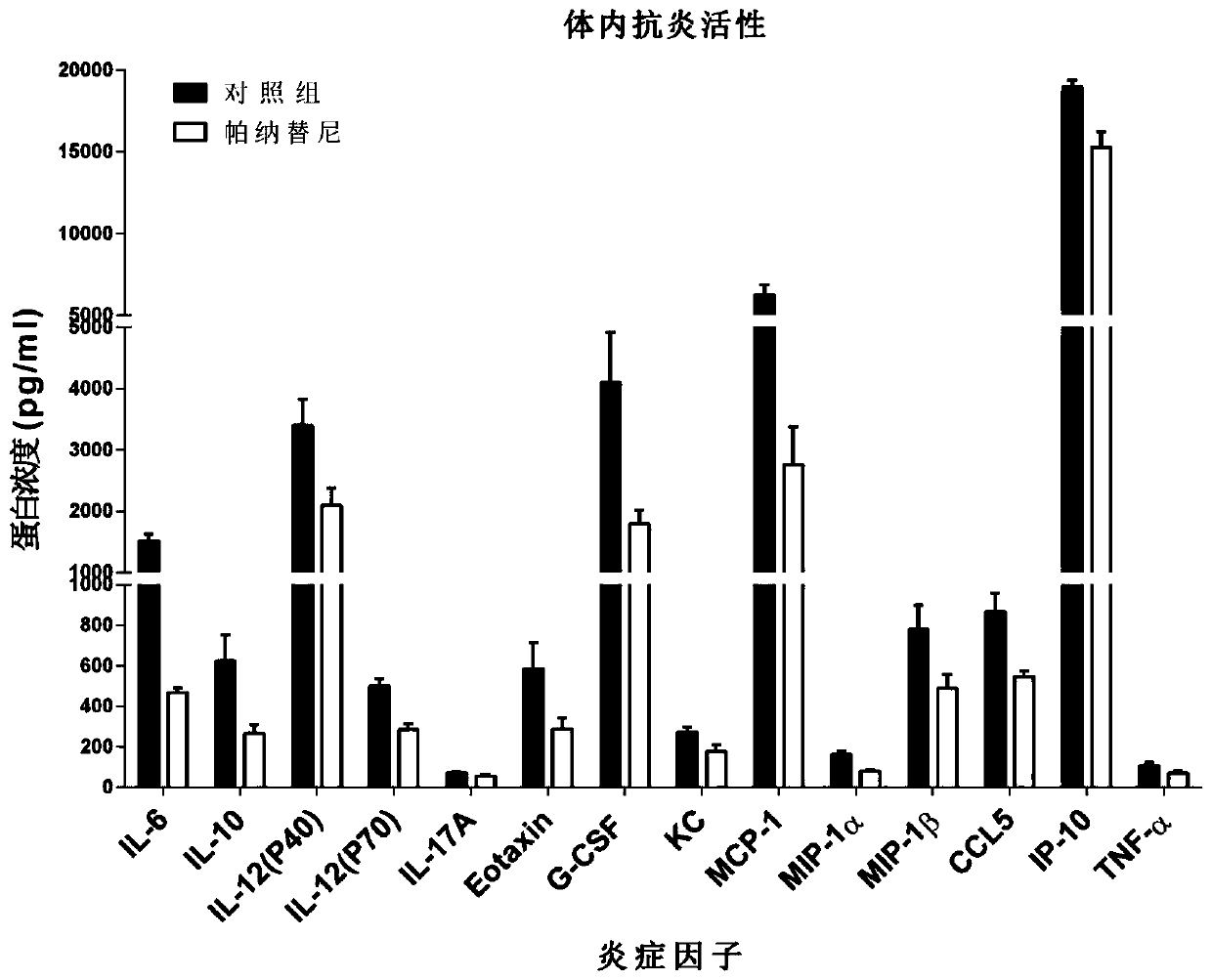
[0067] The results confirmed that ponatinib significantly inhibited the secretion of these three inflammatory factors in a dose-dependent manner, and was a broad-spectrum anti-inflammation drug caused by influenza virus.
Embodiment 3
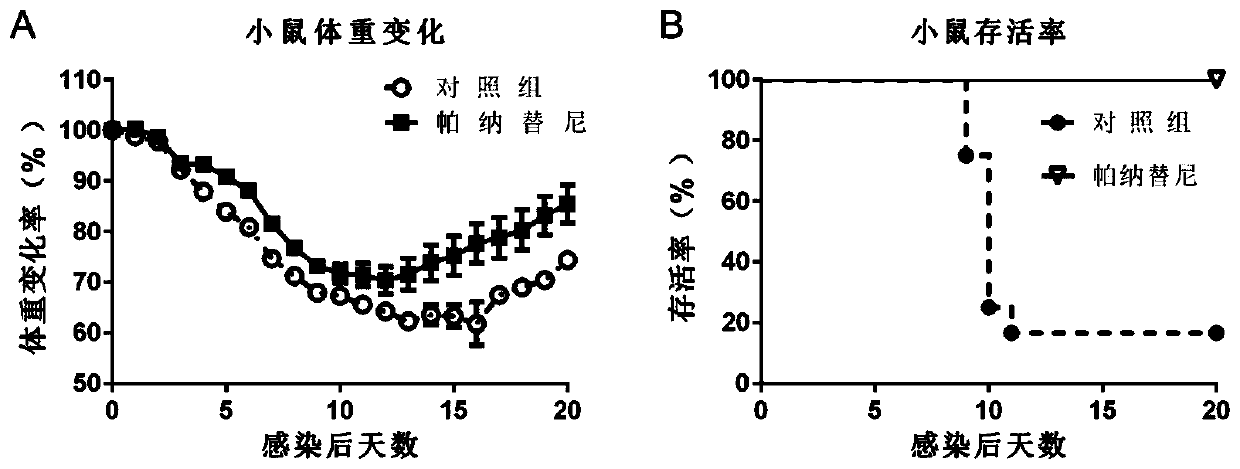
[0068] Example 3: Evaluation of the effect of Ponatinib on anti-influenza virus-induced inflammation in a lethal influenza infection model in mice
[0069] 1 Experimental process:
[0070] 1) BALB / c mice aged 6-8 weeks were randomly divided into a drug evaluation group and a negative control group (PBS), with 8 mice in each group. Before the formal experiment, the mice were acclimated to the environment for 2-3 days.
[0071] 2) On the day of challenge, the mice were lightly anesthetized with 1% pentobarbital sodium (about 0.1ml of anesthetic per gram of body weight), and then infected with 2LD50 H1N1 mouse lung-adapted strain virus solution of 2LD50 by intranasal drip with a pipette gun. .
[0072] 3) Administration started on the 3rd day after virus infection and continued until the 6th day after infection, for a total of 4 days of administration. Oral administration is adopted, and the dose is 15 mg / kg once a day with an interval of 24 hours. The body weight of each gro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com