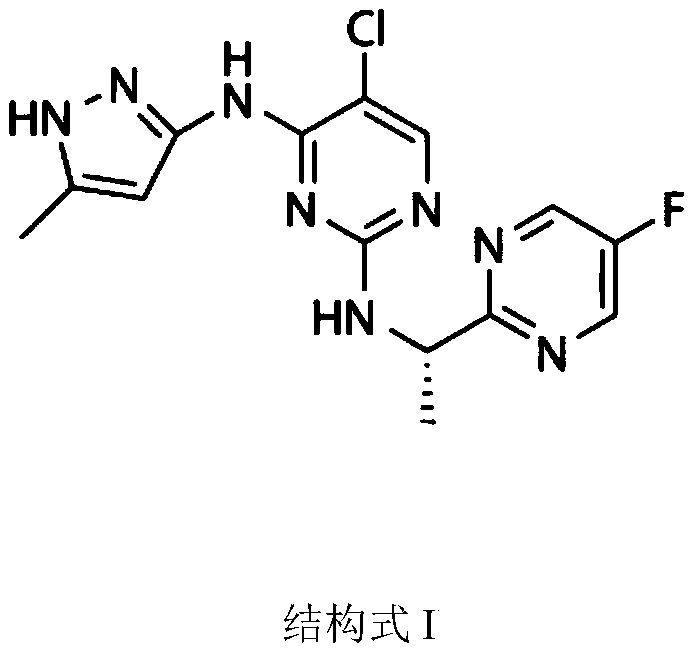
Application of AZD1480 in preparing drug for treating influenza virus infection
A technology of 1.AZD1480, 6.AZD1480, applied in the application field of medicine, can solve the problem of undiscovered AZD1480 anti-therapy influenza, etc., achieve great clinical treatment potential, save cost, improve survival time and ultimate survival rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
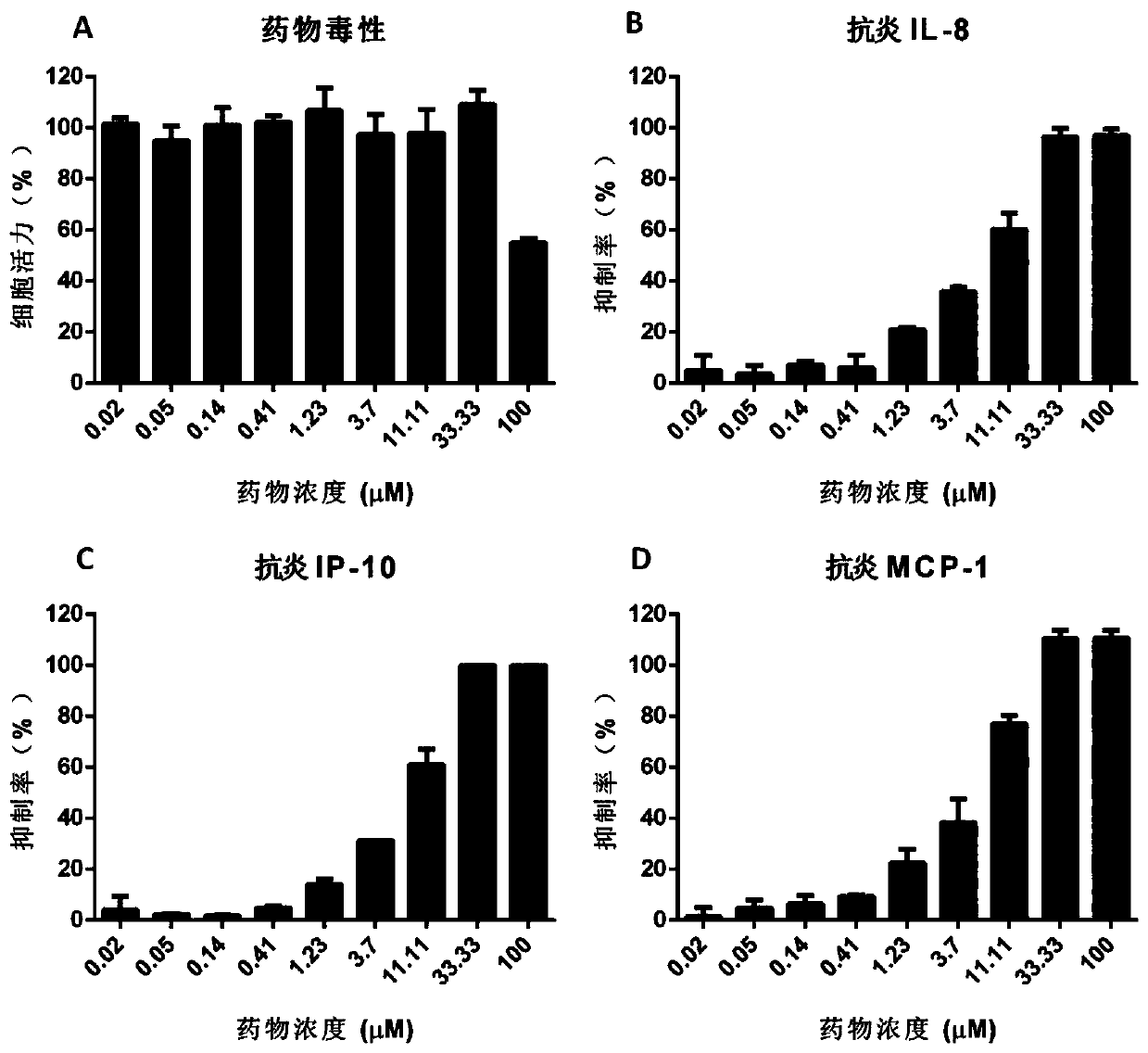
[0048] Example 1: Evaluation of the anti-inflammation activity of AZD1480 in U937 cell line induced by influenza virus
[0049] 1 cell culture
[0050] After 2 passages, the frozen and revived cells were cultured in RPMI-1640 medium containing 10% fetal bovine serum and double antibodies (penicillin 100U / ml, streptomycin 100μg / ml), and the seeding density was not low. at 5x10 5 cell / ml, passage density not higher than 2x10 6 cell / ml.
[0051] 2 Cytotoxicity detection of AZD1480
[0052] U937 cells by 1.5×10 5 Cells / well (volume 100 μL) were seeded in a 96-well cell culture plate; the drug was prepared with 100 μL culture medium (RPMI-1640 medium + 10% serum + double antibody) per well, and added to the corresponding cell wells for mixing. Nine concentration gradients were set for the drug, and two replicate wells were set for each gradient concentration. The final concentrations were 0.02 μM, 0.05 μM, 0.14 μM, 0.41 μM, 1.23 μM, 3.7 μM, 11.11 μM, 33.33 μM and 100 μM. Afte...
Embodiment 2
[0064] Example 2: Evaluation of AZD1480's anti-inflammatory activity against other influenza viruses in U937 cell lines
[0065] In this example, the U937 cell line was infected with the H3N2 subtype (A / Human / Hubei / 3 / 2005), and the method was the same as in Example 1.
[0066] The results confirmed that AZD1480 significantly inhibited the secretion of these three inflammatory factors in a dose-dependent manner, and was a broad-spectrum anti-inflammation drug caused by influenza virus.
Embodiment 3
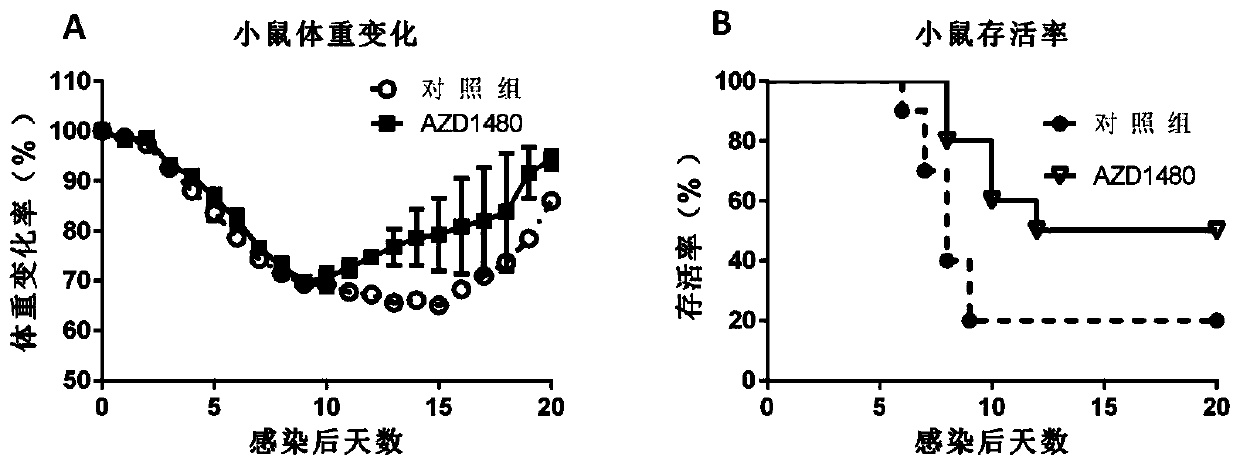
[0067] Example 3: Evaluation of the effect of AZD1480 on anti-inflammation caused by influenza virus in a lethal influenza infection model in mice
[0068] 1 Experimental process:
[0069] 1) BALB / c mice aged 6-8 weeks were randomly divided into a drug evaluation group and a negative control group (PBS), with 8 mice in each group. Before the formal experiment, the mice were acclimated to the environment for 2-3 days.
[0070] 2) On the day of challenge, the mice were lightly anesthetized with 1% pentobarbital sodium (about 0.1ml of anesthetic per gram of body weight), and then infected with 2LD50 H1N1 mouse lung-adapted strain virus solution of 2LD50 by intranasal drip with a pipette gun. .
[0071] 3) Administration started on the 3rd day after virus infection and continued until the 6th day after infection, for a total of 4 days of administration. Oral administration is adopted, the dose is 15mg / kg once a day, with an interval of 24h. The body weight of each group of ani...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com