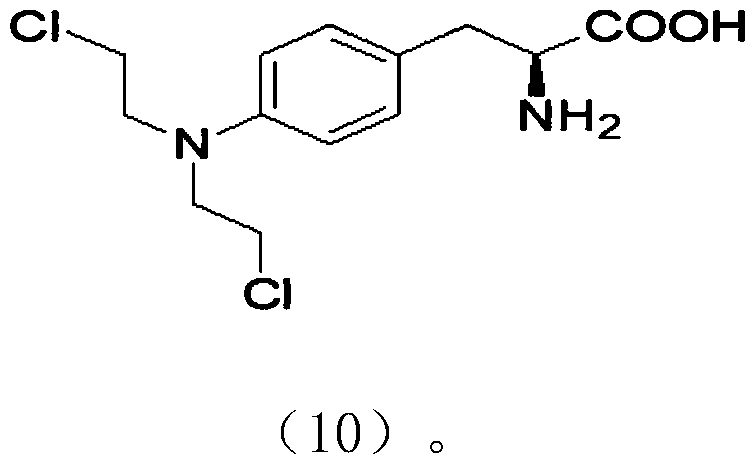
Melphalan intermediate and preparation method thereof
An intermediate and system technology, applied in the field of compound synthesis, can solve problems such as inability to overcome immune evasion, and achieve the effects of improving stereoselectivity, mild reaction conditions and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] A kind of preparation method of Melphalan intermediate is as follows:
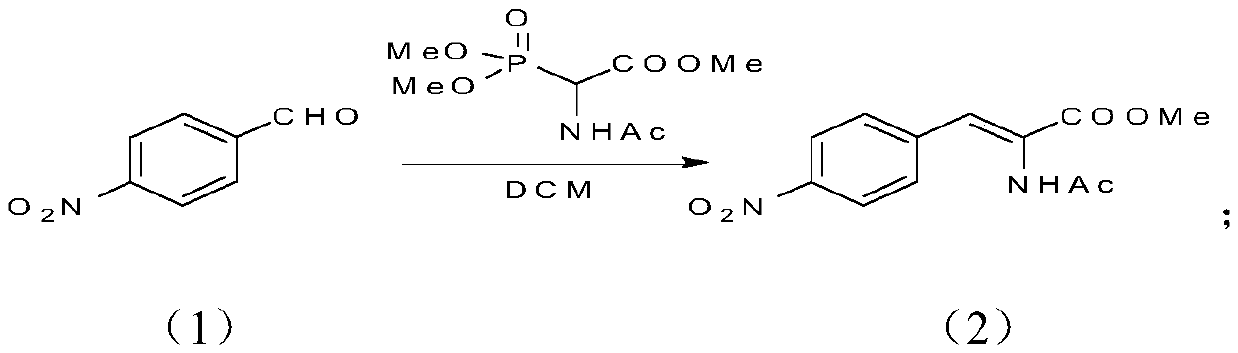
[0045] Step 1:
[0046]
[0047] In a 2L round bottom flask, 165.3g of p-nitrobenzaldehyde was added to 2L of methylene chloride, and 321.2g of methyl 2-acetamido-2-(dimethoxyphosphoryl)acetate was added. Add 237.4g DBU dropwise at 0°C. After the dropwise addition, monitor the reaction for about 2 hours until the raw material disappears. Add 900 mL of 5% sulfuric acid aqueous solution dropwise, filter, and rinse the filter cake with 450 mL of water. Collect the filter cake. After the filter cake was dried, 298.3 g of a yellow solid was obtained, which was the target product. Yield 98.2%, purity 98.1%.
[0048] 1H NMR (400MHz, CDCl3): δ=8.23(d,2H), 7.57(d,2H), 7.42(d,2H), 3.92(s,2H), 2.16(s,3H).
[0049] MS+: 265.1
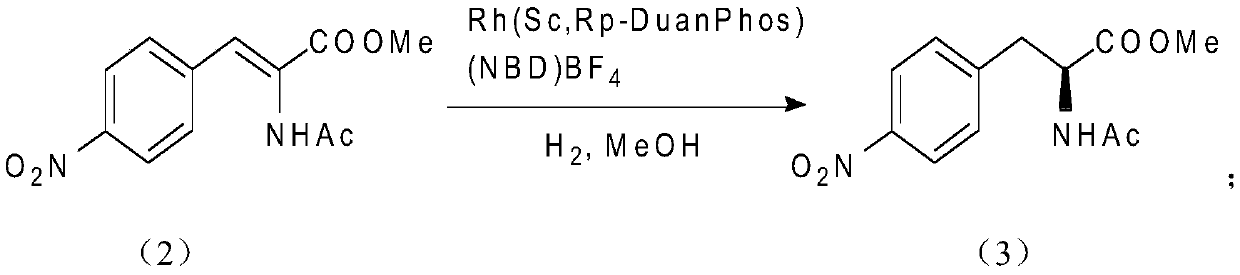
[0050] Step 2:
[0051]
[0052] In a 2000 mL hydrogenation kettle, 237.9 g of the substrate compound 2 and 145 mg of Rh(ScRp-DuanPhos) (NBD) BF4 were added to 1200 mL of me...
Embodiment 2
[0108] A kind of preparation method of Melphalan intermediate is as follows:
[0109] Step 1:
[0110]
[0111] In a 2L round bottom flask, 181.2g of p-nitrobenzaldehyde was added to 2L of methylene chloride, and 344.4g of methyl 2-acetamido-2-(dimethoxyphosphoryl)acetate was added. Add 237.4g of DBU dropwise at 3°C. After the dropwise addition, monitor the reaction for about 2 hours until the raw material disappears. Add 900 mL of 5% sulfuric acid aqueous solution dropwise, filter, and rinse the filter cake with 450 mL of water. Collect the filter cake. After the filter cake was dried, 311.7 g of a yellow solid was obtained, which was the target product. Yield 98.8%, purity 98.9%.
[0112] 1H NMR (400MHz, CDCl3): δ=8.23(d,2H),7.57(d,2H),7.42(d,2H),3.92(s,2H),2.16(s,3H).
[0113] MS+: 265.1
[0114] Step 2:
[0115]
[0116] In a 2000 mL hydrogenation kettle, 237.9 g of the substrate and 145 mg of Rh(ScRp-DuanPhos)(NBD)BF4 were added to 1200 mL of methanol. Under...
Embodiment 3
[0172] A kind of preparation method of Melphalan intermediate is as follows:
[0173] Step 1:
[0174]
[0175] In a 2L round bottom flask, 190.5g of p-nitrobenzaldehyde was added to 2L of methylene chloride, and 344.4g of methyl 2-acetamido-2-(dimethoxyphosphoryl)acetate was added. 237.4g of DBU was added dropwise at 5°C. After the dropwise addition, the reaction was monitored for about 2 hours until the raw material disappeared. Add 900 mL of 5% sulfuric acid aqueous solution dropwise, filter, and rinse the filter cake with 450 mL of water. Collect the filter cake. After the filter cake was dried, 317.5 g of a yellow solid was obtained, which was the target product, compound 2. Yield 98.9%, purity 99.2%.
[0176] 1H NMR (400MHz, CDCl3): δ=8.23(d,2H),7.57(d,2H),7.42(d,2H),3.92(s,2H),2.16(s,3H).
[0177] MS+: 265.1
[0178] Step 2:
[0179]
[0180] In a 2000 mL hydrogenation kettle, 241.2 g of the substrate and 145 mg of Rh(ScRp-DuanPhos) (NBD) BF4 were added to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com