Substituent oxadiazole compound and application thereof
A technology of oxadiazoles and compounds, which is applied in the field of substituted oxadiazoles and can solve the problems that the bactericidal activity of substituted oxadiazoles has not been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
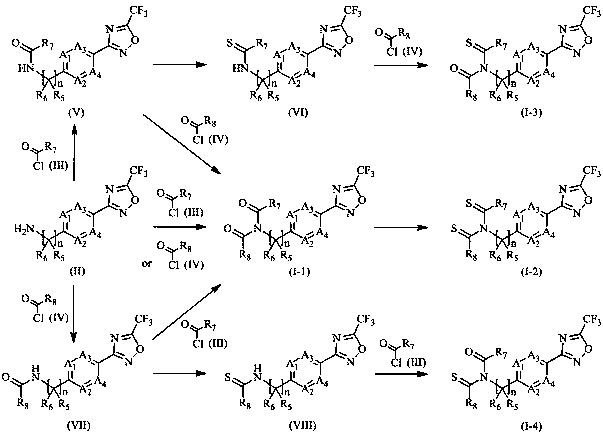
Examples
Embodiment 1
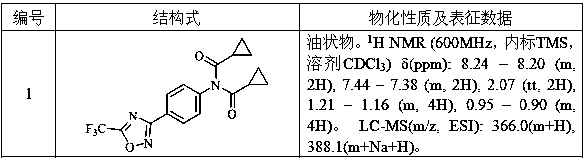
[0154] Embodiment 1: the preparation of compound 1
[0155]
[0156] 0.30 g (1.31 mmol) of 4-(5-trifluoromethyl-1,2,4-oxadiazol-3-yl)aniline (intermediate II-1, can refer to the methods reported in WO2017081309, WO2017081311, WO2017081312, etc. prepared), 0.30 g (2.88mmol)
[0157]Cyclopropylformyl chloride, 0.39 g (3.86 mmol) of triethylamine and 15 ml of dichloromethane were placed in a reaction flask and stirred at room temperature for 5 hours. After the completion of the reaction as monitored by TLC, precipitation under reduced pressure and purification of the residue by column chromatography (eluent is ethyl acetate and petroleum ether, the volume ratio is 1:20~1:10) to obtain 0.43 g of oil, namely compound 1 .
Embodiment 2
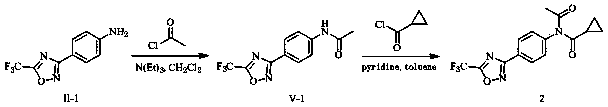
[0158] Embodiment 2: the preparation of compound 2
[0159]
[0160] 0.51 g (2.23 mmol) 4-(5-trifluoromethyl-1, 2, 4-oxadiazol-3-yl) aniline (intermediate II-1), 0.19 g (2.44 mmol) acetyl chloride, 0.27 g (2.67 mmol) of triethylamine and 15 ml of dichloromethane were placed in a reaction flask and stirred at room temperature for 5 hours. After the completion of the reaction as monitored by TLC, precipitation under reduced pressure and purification of the residue by column chromatography (eluent: ethyl acetate and petroleum ether, volume ratio: 1:20~1:10) yielded 0.19 g of a white solid, the intermediate V-1.
[0161] Place 0.19 g (0.70 mmol) of the above-mentioned white solid (intermediate V-1), 0.15 g (1.44 mmol) of cyclopropanecarbonyl chloride, 0.11 g (1.39 mmol) of pyridine and 20 ml of toluene in a reaction flask, and heat up to Reflux and stir the reaction for 8 hours. After the completion of the reaction as monitored by TLC, precipitation under reduced pressure an...
Embodiment 3
[0162] Embodiment 3: the preparation of compound 8
[0163]
[0164] 0.30 g (1.23 mmol) of 4-(5-trifluoromethyl-1, 2, 4-oxadiazol-3-yl) benzylamine (intermediate II-2, can refer to reports such as WO2017055469, WO2017085100, WO2017103219, etc. method), 0.28 g (2.69mmol)
[0165] Cyclopropylformyl chloride, 0.37 g (3.66 mmol) of triethylamine and 15 ml of dichloromethane were placed in a reaction flask and stirred at room temperature for 4 hours. After the completion of the reaction as monitored by TLC, precipitation under reduced pressure and purification of the residue by column chromatography (eluent: ethyl acetate and petroleum ether, volume ratio: 1:20-1:10) yielded 0.41 g of a white solid, namely compound 8 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com