Human mesothelin chimeric antigen receptor, its T cell, its preparation method and application
A chimeric antigen receptor and cell technology, applied in the field of tumor treatment, can solve the problem of restricting the effective activation of specific T cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Example 1. Construction of Mesothelin Recombinant Protein Expression Plasmid
[0083] The cDNA fragment of mesothelin was synthesized in vitro, the HIS tag was added at the end, and the restriction sites EcoR1 and BglII were introduced at both ends, and cloned into the expression vector pCAGGS to construct the recombinant eukaryotic expression plasmid of the full-length mesothelin protein. The above work was completed by Suzhou Synbio.
[0084] The cDNA sequence of mesothelin recombinant protein is as follows:
[0085]ATGTACAGGATGCAACTCCTGTCTTGCATTGCACTAAGTCTT GCACTTGTCACGAATTCGGAAGTGGAGAAGACAGCCTGTCCTTC AGGCAAGAAGGCCCGCGAGATAGACGAGAGCCTCATCTTCTACA AGAAGTGGGAGCTGGAAGCCTGCGTGGATGCGGCCCTGCTGGCC ACCCAGATGGACCGCGTGAACGCCATCCCCTTCACCTACGAGCA GCTGGACGTCCTAAAGCATAAACTGGATGAGCTCTACCCACAAG GTTACCCCGAGTCTGTGATCCAGCACCTGGGCTACCTCTTCCTCA AGATGAGCCCTGAGGACATTCGCAAGTGGAATGTGACGTCCCTG GAGACCCTGAAGGCTTTGCTTGAAGTCAACAAAGGGCACGAAAT GAGTCCTCAGGTGGCCACCCTGATCGACCGCTTTGTGAAGGGAAGGGGCCAGCTAG...
Embodiment 2
[0086] Example 2. Expression and purification of mesothelin protein
[0087] 1) Transfection of HEK293T cells (purchased from Shanghai Cell Bank, BNCC338274): 18 hours before transfection, HEK293T cells were treated with 1.5x10 7 / ml was transferred to 30 15cm petri dishes for culture; 37.5mL DMEM (purchased from Gibco, C11995500CP) (without serum and antibiotics) was transferred to a 50mL tube, and 2970μg polyetherimide (PEI) MegaTran 1.0 (purchased from Alfa Aesar, 9002-98-6) and mix; take 37.5mL DMEM (without serum and antibiotics) to a 50mL tube, add 990μg of mesothelin plasmid DNA and mix; add PEI / DMEM solution to the prepared DNA solution , mix quickly and stand at room temperature for 15 minutes; respectively take 2.5ml PEI / DNA / DMEM mixture into each culture dish and store at 37°C, 5% CO 2 Cultivated in an incubator. After 6 hours of transfection, carefully aspirate the culture medium, and add 25ml of new culture medium DMEM+2%FBS (purchased from Gibco, 10270)+0.12% d...
Embodiment 3
[0091] Example 3. Preparation and preliminary screening of mesothelin monoclonal antibody
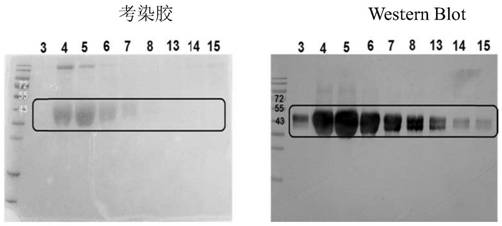
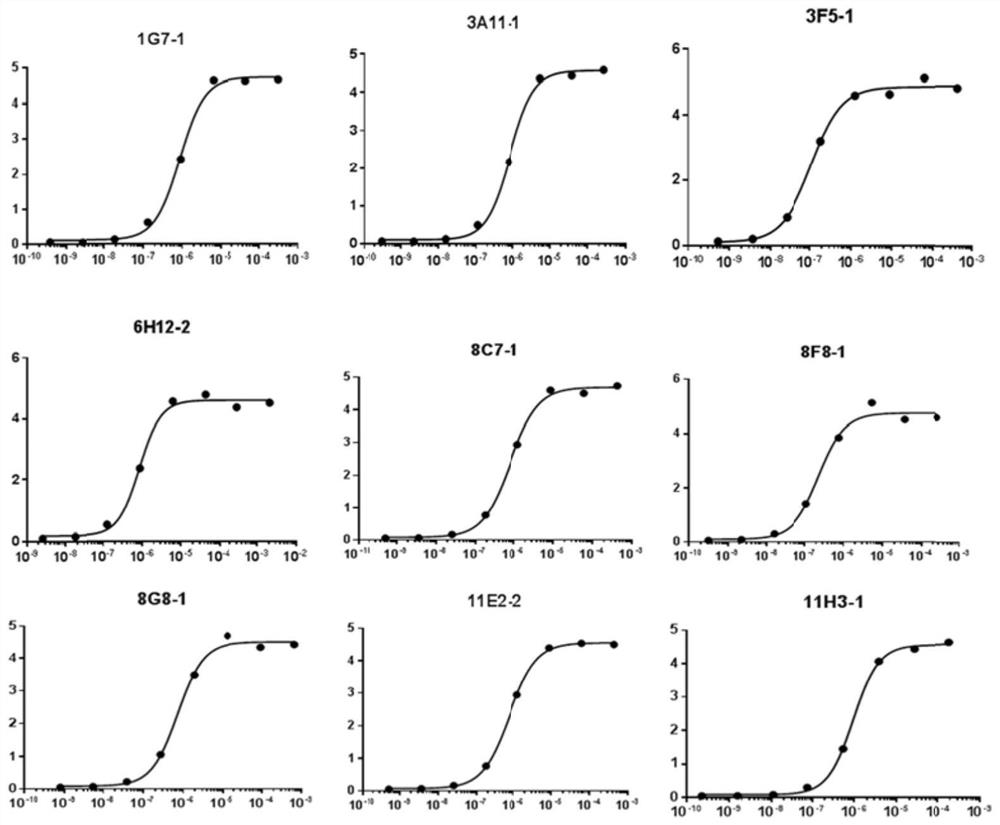
[0092] This part of the work was completed by Nanjing GenScript Company. The purified full-length mesothelin recombinant protein (hereinafter referred to as mesothelin antigen) obtained in Example 2 was used to immunize B6 / C57 mice according to standard methods. Fusion, screening, etc., to obtain 9 monoclonal strains.
[0093] The corresponding fusion plate cell lines are 1G7-1, 3A11-1, 3F5-1, 6H12-2, 8C7-1, 8F8-2, 8G8-2, 11E2-2, 11H3-2, and the corresponding monoclonal antibodies are labeled with this .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com