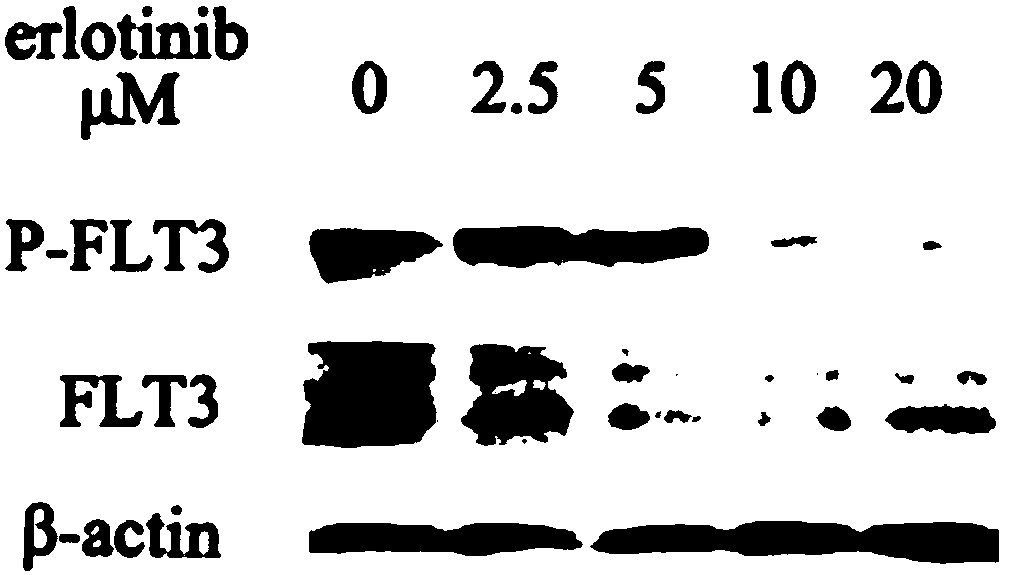Application of erlotinib in preparation of FLT3 inhibitor-type medicine
An inhibitor and drug technology, which can be used in drug combinations, antitumor drugs, pharmaceutical formulations, etc., can solve the problem of no erlotinib inhibiting FLT3 enzyme activity, and achieve good clinical application prospects, good effects, and strong inhibitory effects. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1, the enzymatic level inhibitory activity of erlotinib to FLT3 kinase
[0028] 1. Experimental materials
[0029] Liquid container, 384 plate, multi-label microplate detector (Perkin Elmer), KinEASETM and FMS-like tyrosine kinase 3 (FLT3) were purchased from Beijing Yiqiao Shenzhou.
[0030] 2. Experimental method
[0031] The experiment was operated according to the optimization program provided in the HTRF kit, and the experimental data were fitted by Graphpad software.
[0032] 3. Experimental results
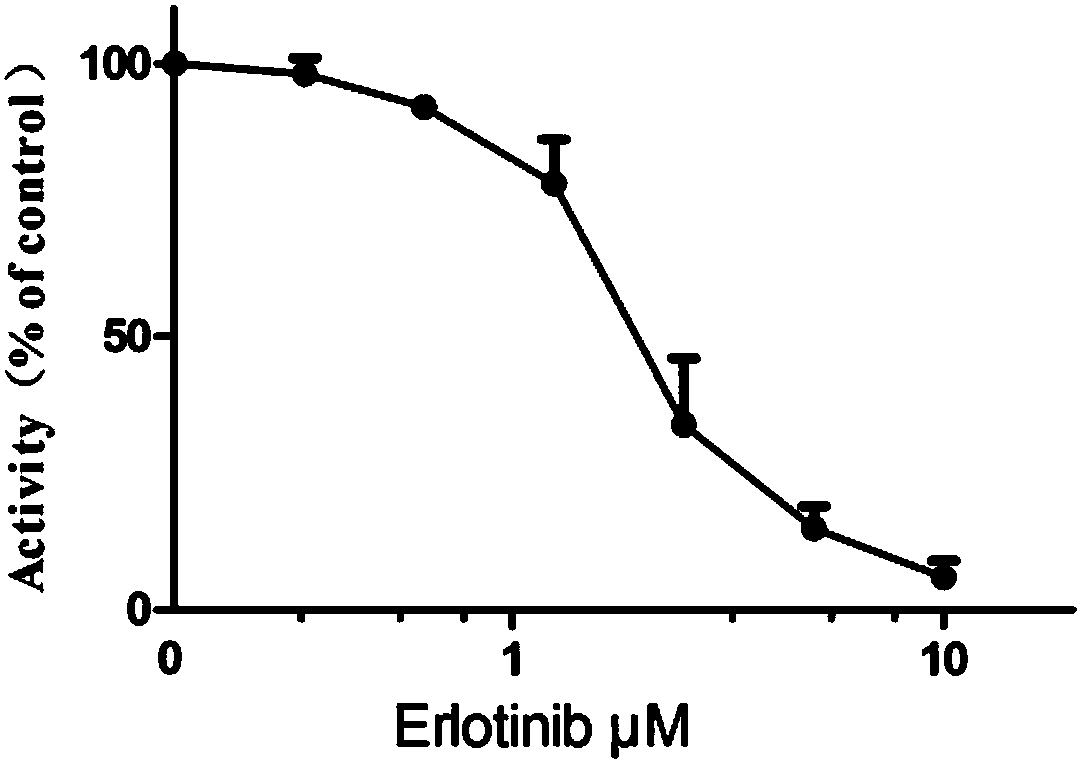
[0033] From figure 1 It can be seen that in the enzyme level activity test, erlotinib exhibited a significant inhibitory effect on FLT3, indicating that the drug can produce corresponding pharmacodynamic effects by inhibiting the activity of FLT3.
Embodiment 2
[0034] Example 2, Erlotinib inhibits FLT3 activity in MV4-11 cells
[0035] 1 Experimental materials
[0036] 1.1 Main reagents
[0037] IMDM medium, fetal calf serum, and trypsin were purchased from Gibco (Life technologies corporation).
[0038] FLT3 and p-FLT3 antibodies were purchased from cell signaling technology company.
[0039] 1.2 Cell lines
[0040] The MV4-11 cell line was purchased from ATCC, USA.
[0041] 2 Experimental methods
[0042] After the MV4-11 cells were treated with gradient concentrations of erlotinib (20, 10, 5, 2.5 μM) for 20 h, the cells were collected by centrifugation and lysed with RIPA to extract the total intracellular protein. Then, the protein was separated by SDS-PAGE electrophoresis separation and PVDF membrane transfer and transferred to PVDF membrane. Subsequently, they were blocked by FBS, immunocombined with FLT3 or p-FLT3 antibody, and incubated with secondary antibody. Finally, the effects of different concentrations of erloti...
Embodiment 3
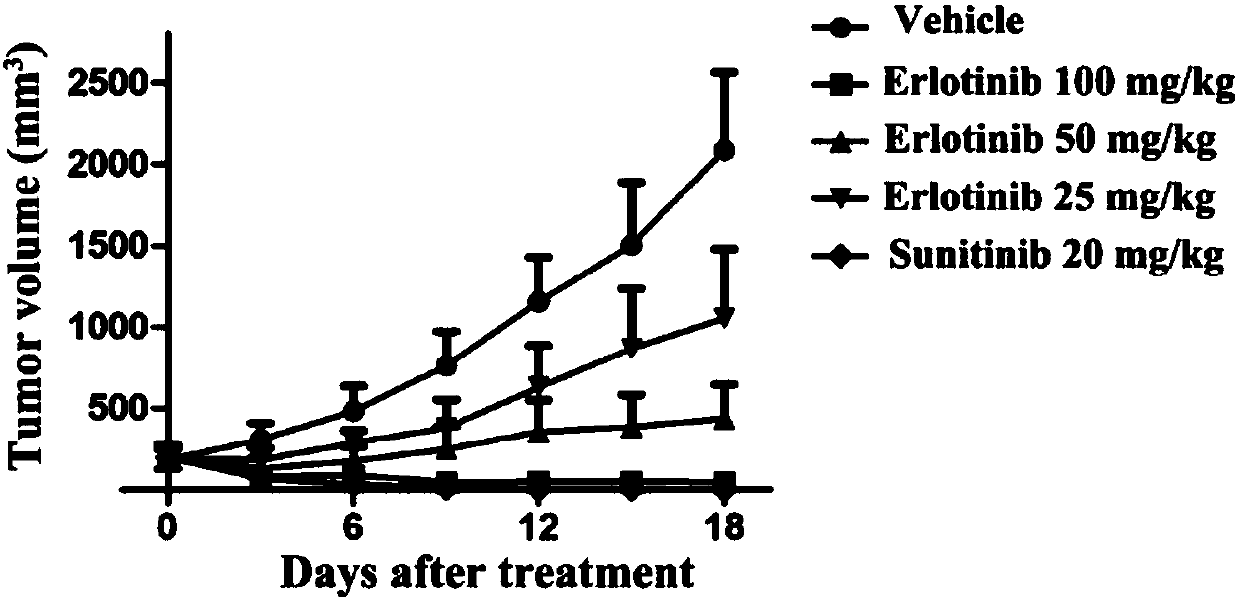
[0045] Embodiment 3, in vitro anti-tumor cell proliferation experiment of erlotinib
[0046] 1 Experimental materials
[0047] 1.1 Main reagents
[0048] RPMI-1640 and IMDM medium, fetal calf serum, and trypsin were purchased from Gibco (Life technologies corporation).
[0049] Dimethylsulfoxide (DMSO) and tetramethylazolium salt (MTT) were purchased from Sigma (USA).
[0050] Erlotinib was purchased from Nanjing Kangmanlin Technology Co., Ltd., and was prepared into a 20 mg / ml storage solution with 100% DMSO during in vitro experiments. It was stored in a -20°C refrigerator in the dark for future use, and was diluted to the required concentration with complete culture medium before use.
[0051] 1.2 Cell lines and culture
[0052] MV4-11 cell line, MOLM-13 cell line, and IL3-dependent proto-B lymphocyte Ba / F3 were purchased from ATCC in the United States, and Ba / F3-FLT3-ITD cells expressing FLT3-ITD protein were donated by Professor Yang Shengyong of Sichuan University . ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com