A kind of synthetic method of nitrendipine
A technique of nitrendipine and a synthetic method, which is applied in the field of material synthesis, can solve the problems of reducing the synthesis efficiency of the target product, reducing the purity of the target product, etc., and achieve the effects of excellent solvent performance, improving the purity of the product, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
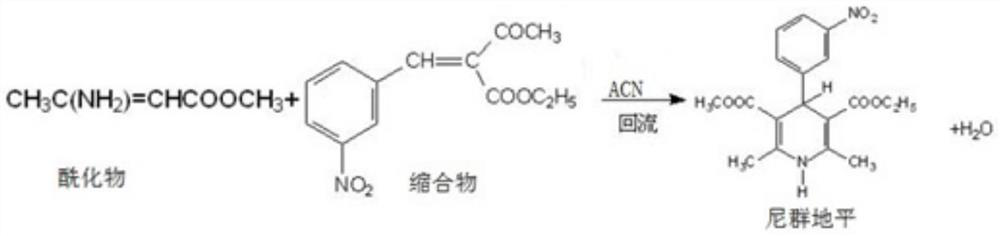
[0023] A kind of synthetic method of nitrendipine of the present embodiment, comprises the following steps:
[0024] S1 adds 750g acetonitrile in the there-necked flask;
[0025] S2 stirred acetonitrile, added nitrendipine acylate 200g, nitrendipine condensate 450g successively;
[0026] S3 slowly heats up and reacts at 75°C for 4.5 hours;
[0027] After the S4 reaction is completed, slowly cool down to 5°C and crystallize for 4-6 hours;
[0028] S5 filters nitrendipine, and washes the filter cake with a small amount of cold methanol-water mixed solution (methanol:water=1:1);
[0029] S6 dried nitrendipine at 70° C. for 6 hours to obtain 530 g of nitrendipine with a yield of 86%.
[0030]
Embodiment 2
[0032] The features of this embodiment that are the same as those of Embodiment 1 will not be described in detail. The features of this embodiment that are different from Embodiment 1 are:
[0033] A kind of synthetic method of nitrendipine of the present embodiment, comprises the following steps:
[0034] S1 adds 600g acetonitrile in the there-necked flask;
[0035] S2 stirs acetonitrile, adds nitrendipine acylate 80g, nitrendipine condensate 180g successively;
[0036] S3 slowly heats up and reacts at 80°C for 4.5 hours;
[0037] After the S4 reaction is completed, slowly cool down to 0°C and crystallize for 4-6 hours;
[0038] S5 filters nitrendipine, and washes the filter cake with a small amount of cold methanol-water mixed solution (methanol:water=1:1);
[0039] S6 Dry the nitrendipine at 80° C. for 6 hours.
Embodiment 3
[0041] The features of this embodiment that are the same as those of Embodiment 1 will not be described in detail. The features of this embodiment that are different from Embodiment 1 are:
[0042] A kind of synthetic method of nitrendipine of the present embodiment, comprises the following steps:
[0043] S1 adds 600g acetonitrile in the there-necked flask;
[0044] S2 stirs acetonitrile, adds nitrendipine acylate 120g, nitrendipine condensate 270g successively;
[0045] S3 heats up slowly and reacts at 78°C for 4.5 hours;
[0046] After the S4 reaction is completed, slowly cool down to 2°C and crystallize for 4-6 hours;
[0047] S5 filters nitrendipine, and washes the filter cake with a small amount of cold methanol-water mixed solution (methanol:water=1:1);
[0048] S6 Dry the nitrendipine at 75° C. for 6 hours.
[0049] Because alcohols are used as reaction solvents, the nitrendipine synthesized after the reaction does not reach the Pharmacopoeia standard, and secondar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com