A kind of detection method of azobisisobutylamidine dihydrochloride
A technology of azobisisobutylamidine dihydrochloride and a detection method, which is applied in the field of drug residue detection, can solve problems such as poor stability and failure to meet the acceptable range of methodological verification, and achieve easy operation and ensure drug safety , The effect of simple detection process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The preparation method of the test product (colesevelam) refers to PCT patent WO95 / 34585.
[0042] (1) Solution preparation:
[0043] Mobile phase A: 0.1% phosphoric acid aqueous solution (containing 5mmol / L sodium octane sulfonate);
[0044] Mobile phase B: acetonitrile;
[0045] Preparation solution C: Dissolve an appropriate amount of chloride salt in 1000ml of purified water;
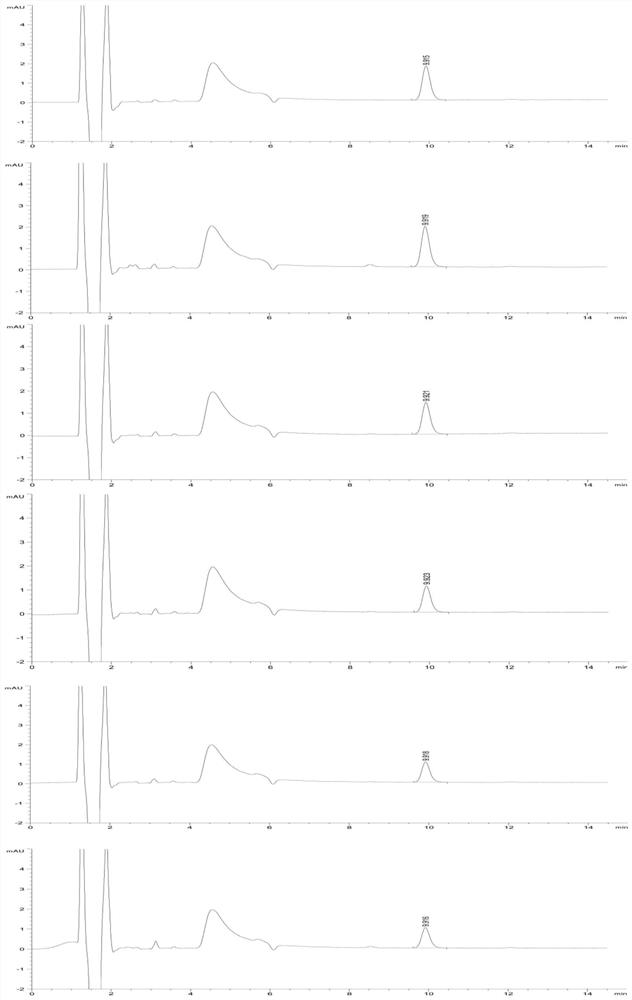
[0046] (2) Preparation of reference substance solution: take about 100mg of V-50 reference substance, weigh it accurately, put it in a 100ml volumetric flask, add preparation solution C to dissolve and dilute to the mark, shake well, and prepare 1mg of V-50 per 1ml Precisely measure 1ml, put it in a 100ml volumetric flask, add preparation solution C to dissolve and dilute to the mark, shake well, and prepare a solution containing 0.01mg V-50 per 1ml; precisely measure 1ml, put it in a 100ml volumetric flask , add preparation solution C to dissolve and dilute to the mark, shake well, and pr...
Embodiment 2
[0072] The HPLC chromatographic condition of this part embodiment is with embodiment 1, and difference is:
[0073] (1) The preparation solution C solution is prepared according to the ratio in the table;
[0074] The test results of Example 2 are shown in Table 2.
[0075] Table 2
[0076]
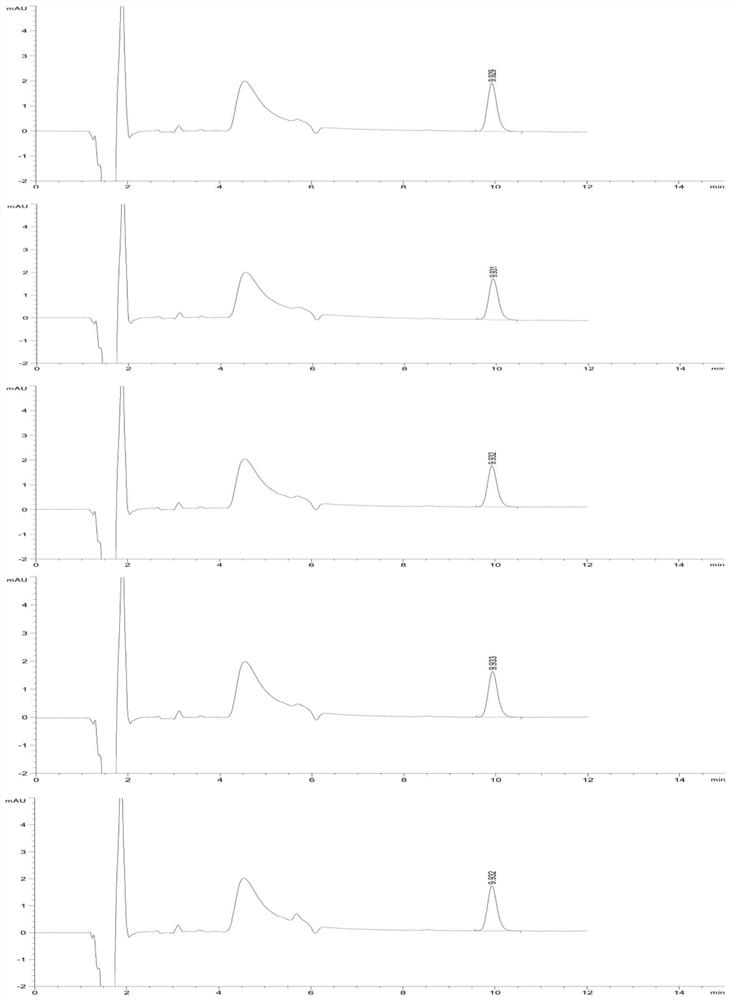
[0077] Studies have shown that when the concentration of chloride salt in the solution is 10-40mmol / L, the maximum change RSD of the peak area of the reference solution within the specified time is less than 10%, and the residual V-50 detection results in the test sample are basically the same. Meet inspection requirements and relevant regulatory requirements.
Embodiment 3
[0079] The HPLC chromatographic condition of this part embodiment is with embodiment 1, and difference is:
[0080] (1) The preparation solution C solution is prepared according to the ratio in the table;
[0081] (2) HPLC measurement: At the time points of 0h, 2h, 4h, 6h, and 8h, draw an equal volume of reference substance solution and inject it into a high-performance liquid chromatograph for measurement
[0082] (3) Calculation formula:
[0083]
[0084] In the formula, A n is the peak area when n;
[0085] A 0 Peak area at 0;
[0086] The test results of Example 3 are shown in Table 3.
[0087] table 3
[0088]
[0089] The research results show that even if the concentration limit of the V-50 reference substance solution is as low as 0.01 μg / ml, the sample solution prepared with a chloride salt solution as a medium can also slow down the decomposition of V-50 in aqueous solution, and the peak area within 8 hours The change rate is less than 5%, which is far l...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com