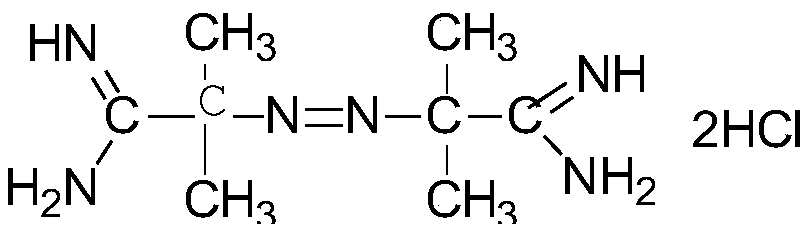
Method for detecting azobis(isobutylamidine hydrochloride)
A technology of azobisisobutyramide dihydrochloride and diisobutyramide dihydrochloride, which is applied in the field of detection of azobisisobutyramide dihydrochloride, can solve the problems of poor stability and non-compliance with methodological verification. Accept the range and other issues to achieve the effect of simple detection process, ensuring drug safety and strong practicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The preparation method of the test product (colesevelam) refers to PCT patent WO95 / 34585.
[0042] (1) Solution preparation:
[0043] Mobile phase A: 0.1% phosphoric acid aqueous solution (containing 5mmol / L sodium octane sulfonate);
[0044] Mobile phase B: acetonitrile;
[0045] Preparation solution C: Dissolve an appropriate amount of chloride salt in 1000ml of purified water;
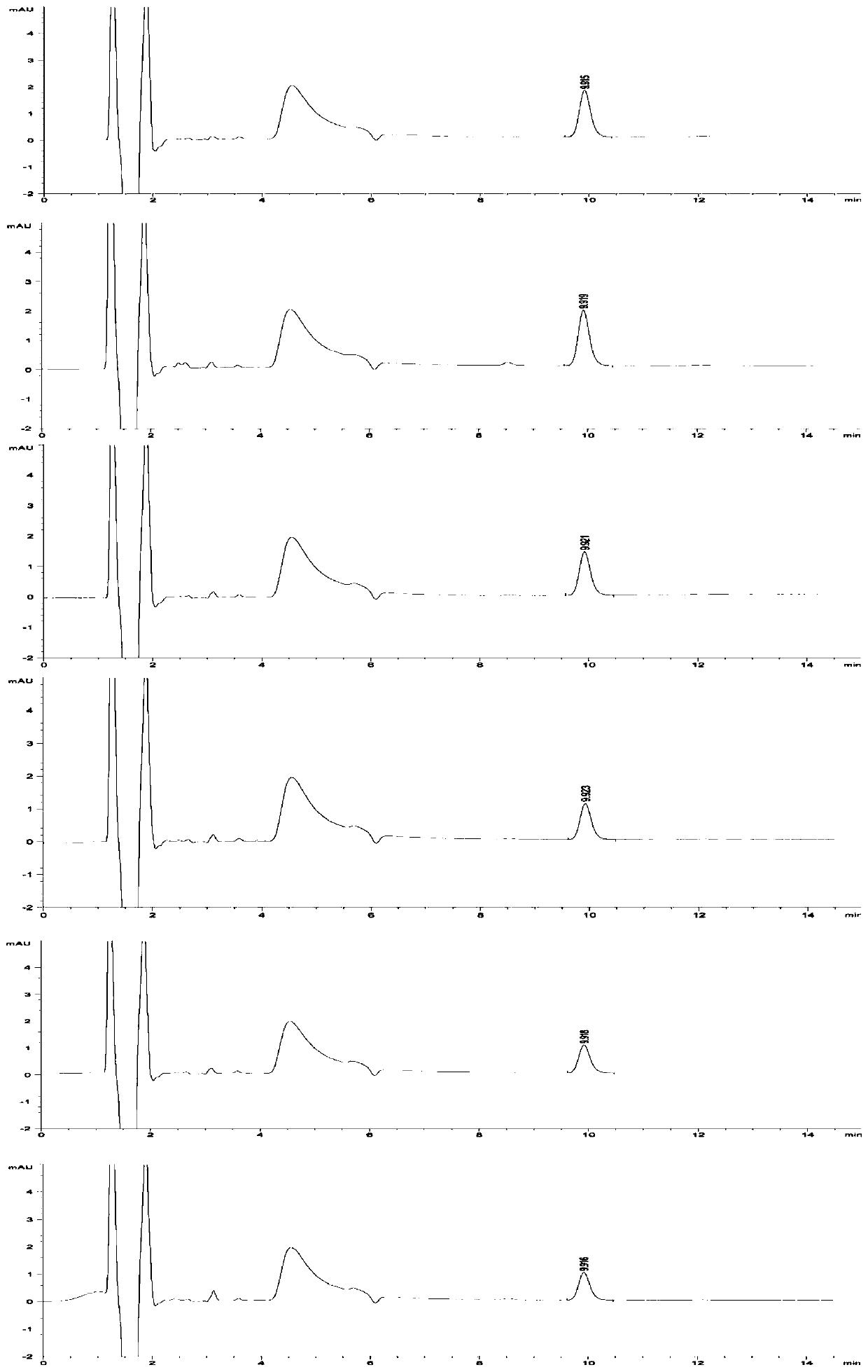
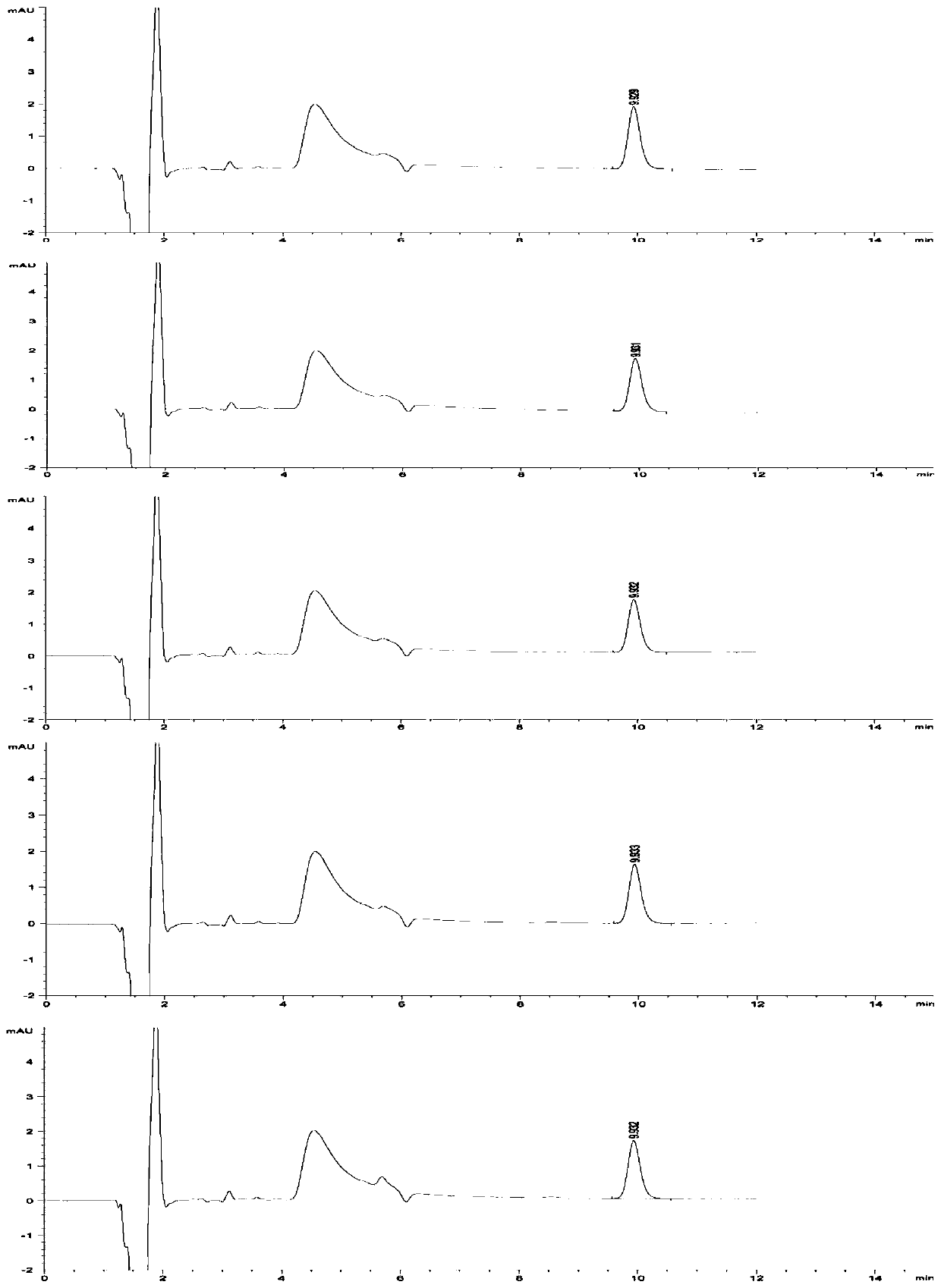
[0046] (2) Preparation of reference substance solution: take about 100mg of V-50 reference substance, weigh it accurately, put it in a 100ml volumetric flask, add preparation solution C to dissolve and dilute to the mark, shake well, and prepare 1mg of V-50 per 1ml Precisely measure 1ml, put it in a 100ml volumetric flask, add preparation solution C to dissolve and dilute to the mark, shake well, and prepare a solution containing 0.01mg V-50 per 1ml; precisely measure 1ml, put it in a 100ml volumetric flask , add preparation solution C to dissolve and dilute to the mark, shake well, and pr...
Embodiment 2
[0072] The HPLC chromatographic condition of this part embodiment is with embodiment 1, and difference is:
[0073] (1) The preparation solution C solution is prepared according to the ratio in the table;
[0074] The test results of Example 2 are shown in Table 2.
[0075] Table 2
[0076]
[0077] Studies have shown that when the concentration of chloride salt in the solution is 10-40mmol / L, the maximum change RSD of the peak area of the reference solution within the specified time is less than 10%, and the residual V-50 detection results in the test sample are basically the same. Meet inspection requirements and relevant regulatory requirements.
Embodiment 3
[0079] The HPLC chromatographic condition of this part embodiment is with embodiment 1, and difference is:
[0080] (1) The preparation solution C solution is prepared according to the ratio in the table;
[0081] (2) HPLC measurement: At the time points of 0h, 2h, 4h, 6h, and 8h, draw an equal volume of reference substance solution and inject it into a high-performance liquid chromatograph for measurement
[0082] (3) Calculation formula:
[0083]
[0084] In the formula, A n is the peak area when n;
[0085] A 0 Peak area at 0;
[0086] The test results of Example 3 are shown in Table 3.
[0087] table 3
[0088]
[0089] The research results show that even if the concentration limit of the V-50 reference substance solution is as low as 0.01 μg / ml, the sample solution prepared with a chloride salt solution as a medium can also slow down the decomposition of V-50 in aqueous solution, and the peak area within 8 hours The change rate is less than 5%, which is far l...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com