Continuous flow preparation method of 2,6-diethyl-4-methylaniline diazonium salt
A technology of methylaniline and diethyl, applied in the field of preparation of pesticide intermediates, can solve problems such as increased energy consumption, increased production capacity of diazonium salts, and potential safety hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
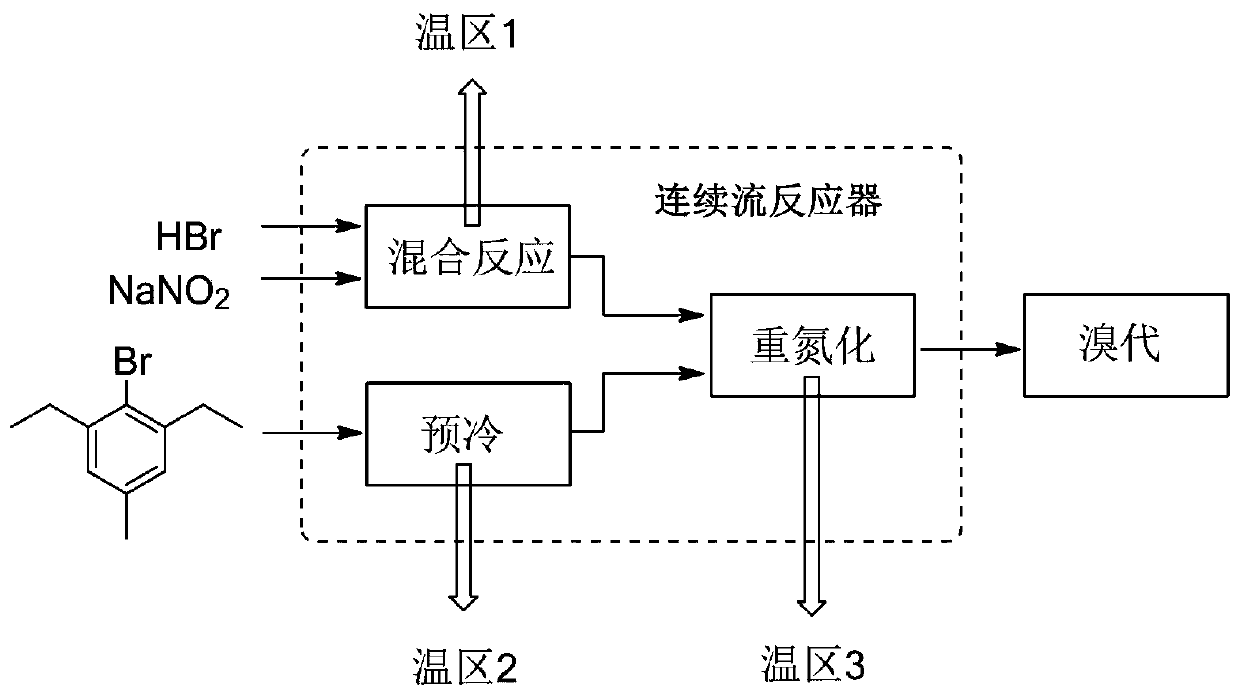
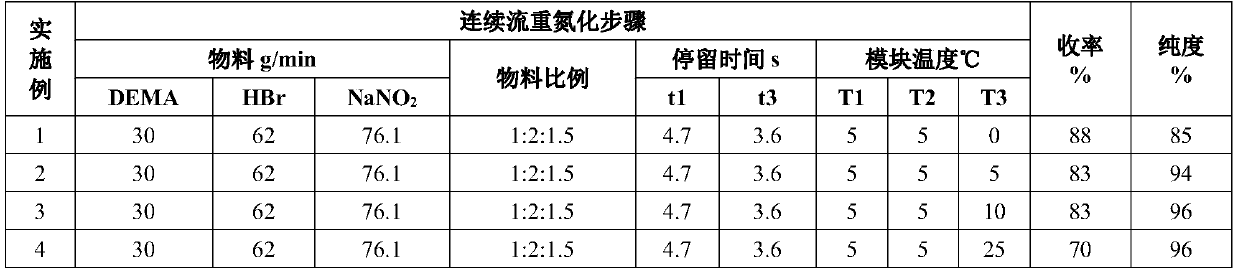
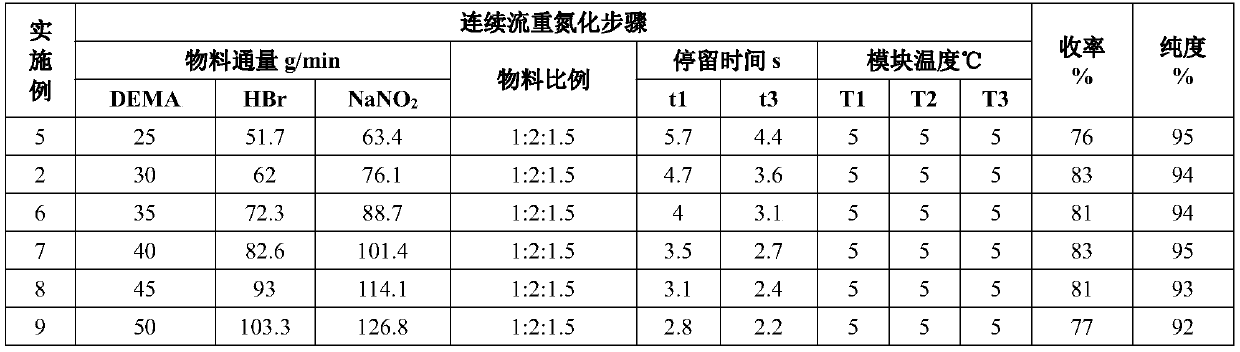
[0073] Such as figure 1 As shown, the concentration of 48wt% HBr aqueous solution and the concentration of 25wt% NaNO 2 The aqueous solution is passed into the pre-cooling module (temperature zone 1) for mixing and reaction, and another 2,6-diethyl-4-methylaniline is passed into the temperature zone 2 for pre-cooling. The material flowing through temperature zone 1 and the material whose temperature is pre-controlled in temperature zone 2 are mixed in temperature zone 3, and flow through temperature zone 3, where the diazotization reaction is completed to generate a diazonium salt intermediate.
[0074] After the diazonium salt intermediate leaves the reactor, it is directly connected to the bromination reaction kettle to carry out the bromination reaction at 80°C. According to the amount of 2,6-diethyl-4-methylaniline that is continuously introduced, add 0.5eq FeSO 4 .7H 2 O, 1.18eq 48% HBr, 3eq NaBr. After the bromination reaction is finished, add methylcyclohexane to ex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com