Method for determining chloral hydrate content by high performance liquid chromatography
A chloral hydrate and high-performance liquid chromatography technology, which is applied in the field of chloral hydrate content, can solve the problems of inaccurate chloral hydrate, lack of specificity and accuracy, and achieve the goals of saving organic solvents, easy operation, and cost reduction Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] (1) Preparation of reference substance solution and need testing solution: accurately weigh an appropriate amount of chloral hydrate reference substance, dissolve it with mobile phase to make a reference substance solution of specified concentration; accurately weigh an appropriate amount of chloral hydrate test substance, and Dissolve or dilute and constant volume, obtain need testing solution; Wherein said mobile phase is acetonitrile-water;
[0024] (2) Determination: The reference substance solution and need testing solution are carried out liquid chromatography detection, record chromatogram, according to reference substance solution concentration, peak area and need testing solution peak area, external standard method calculates in the need testing solution Chloral hydrate content.
[0025] In some embodiments, the calculation formula is as follows:
[0026]
[0027] in
[0028] A 供试品 refers to the peak area of the test solution;
[0029] A 对照品 refers to...
Embodiment 1
[0081] In the present embodiment, measure the content of chloral hydrate through the following steps:
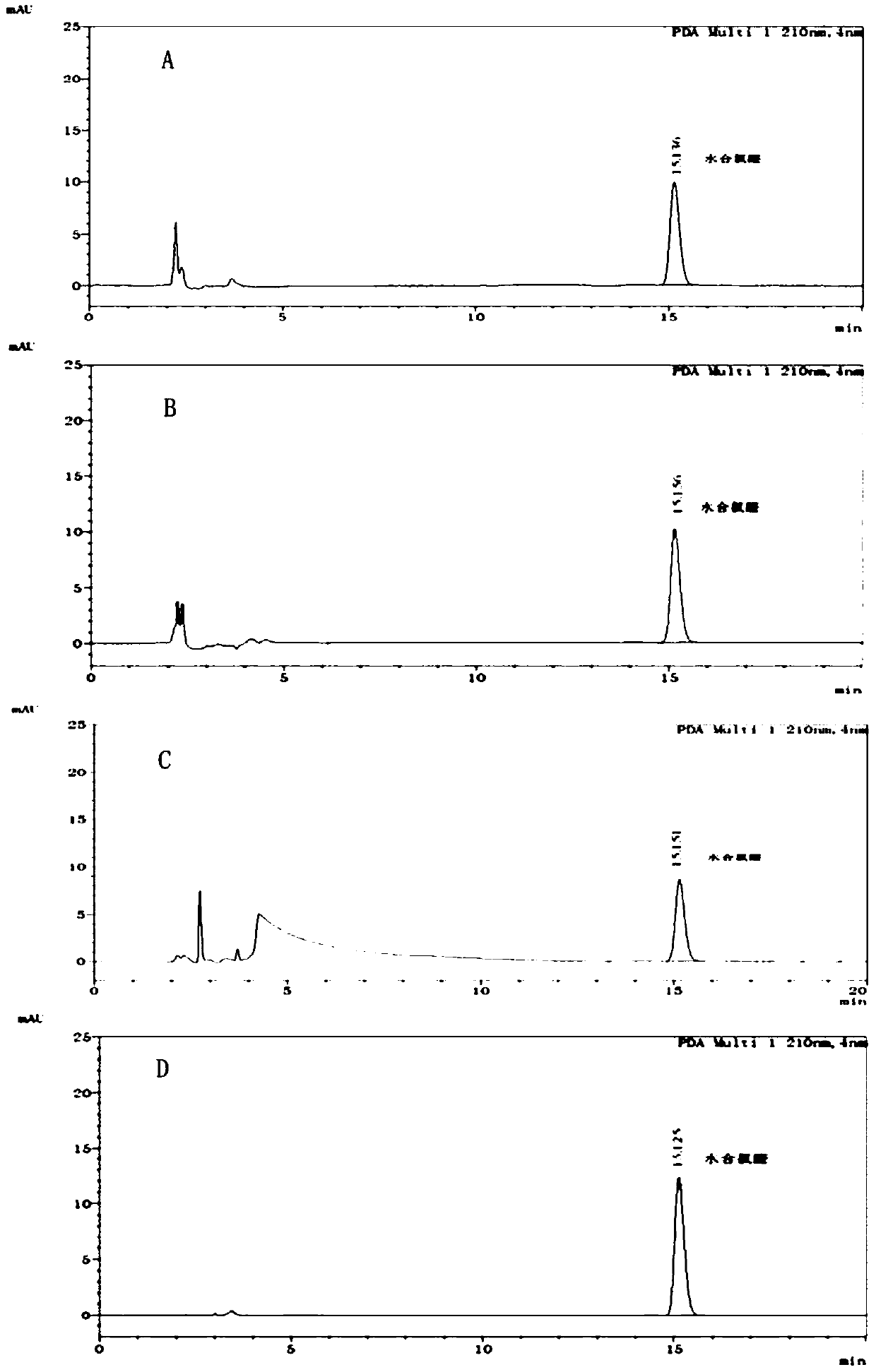
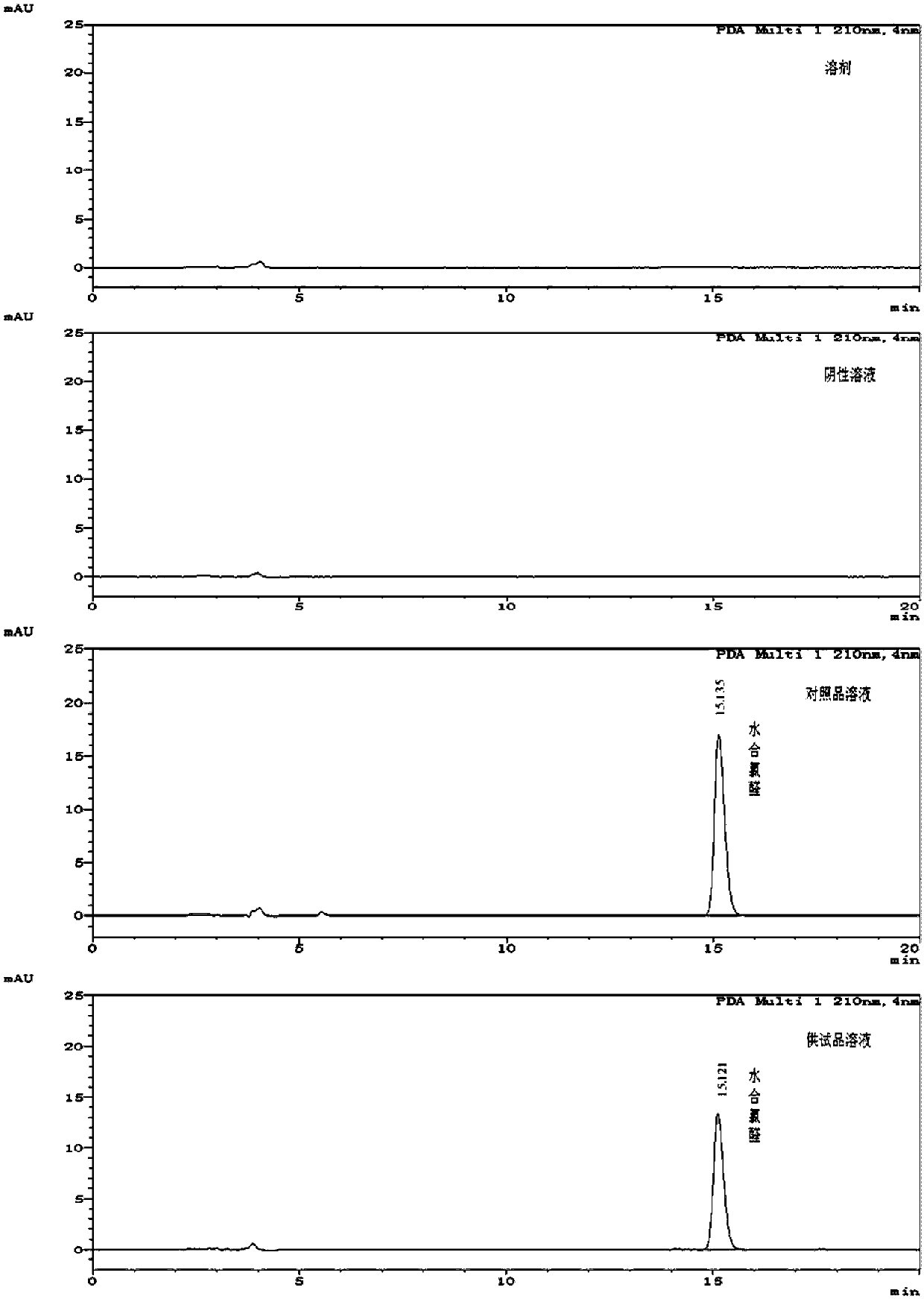
[0082] (1) Preparation of reference substance solution and need testing solution: Accurately weigh 25.02 mg of chloral hydrate reference substance, put it in a 25ml measuring bottle, add mobile phase (acetonitrile: water=10:90) to dissolve and dilute to the scale, as Reference solution (C 对照品 =1.0128mg / ml); Precision takes by weighing chloral hydrate crude drug 23.63mg, puts in 25ml measuring bottle, adds mobile phase (acetonitrile: water=10: 90) to dissolve and be diluted to scale, obtain need testing solution (chlorine hydrate The aldehyde concentration is about 1 mg / ml);
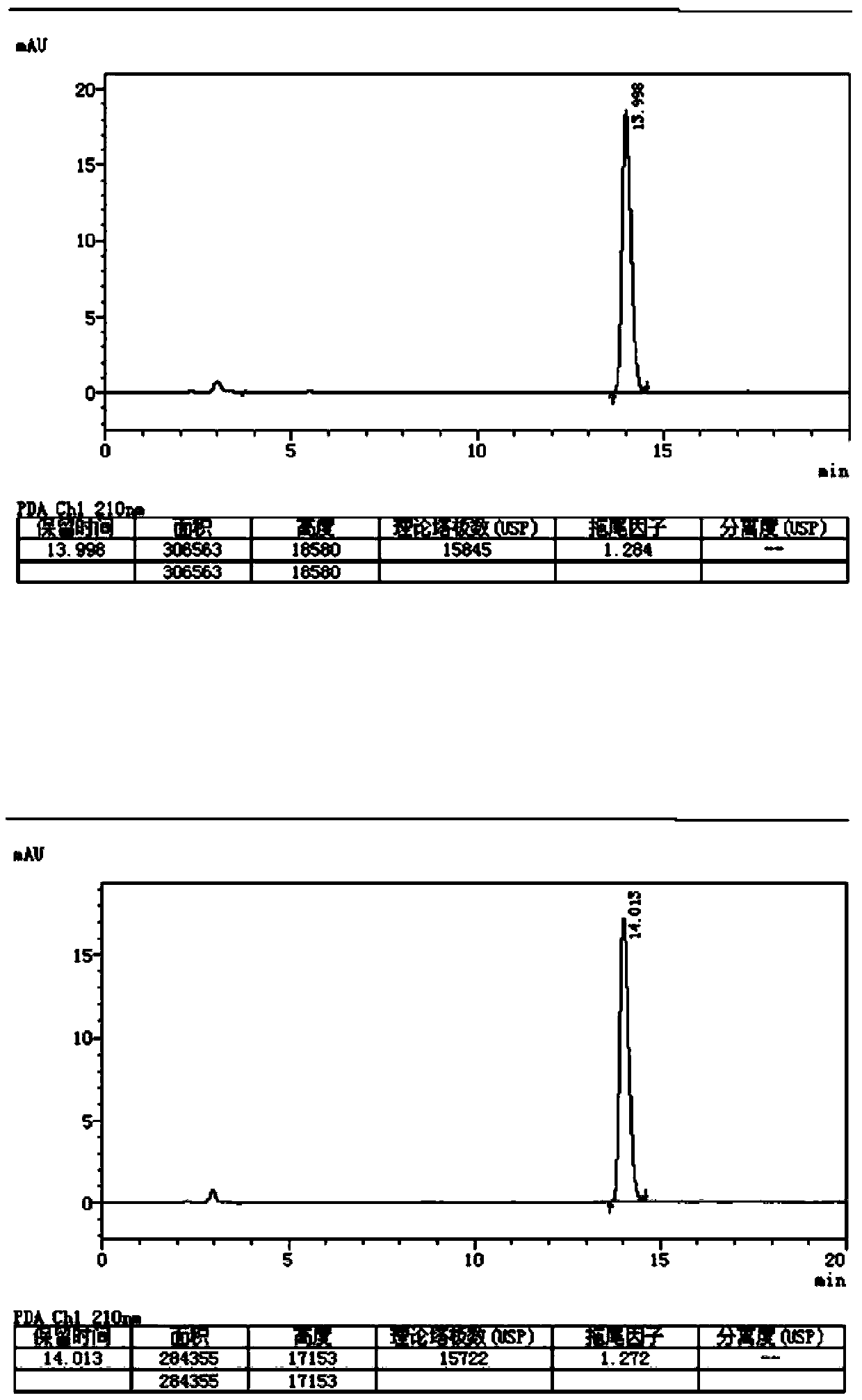
[0083] (2) measure: described reference substance solution and need testing solution are measured according to following chromatographic conditions, record chromatogram (such as figure 1 Shown), according to reference substance solution concentration, peak area and need testing product peak area, calc...
Embodiment 2
[0096] Example 2: Methodological Validation
[0097] According to the "Chinese Pharmacopoeia" 2015 edition appendix (9101) drug quality standard analytical method validation guidelines and ICH analytical method validation guidelines for methodological validation, the process and results are as follows:
[0098] Solution preparation
[0099] Preparation of reference stock solution
[0100] Take about 0.1 g of the chloral hydrate reference substance, accurately weigh it, put it in a 50ml measuring bottle, add mobile phase to dissolve and dilute to the mark, shake well, and use it as the reference substance stock solution.
[0101] Preparation of reference solution
[0102] Take about 25mg of chloral hydrate reference substance, weigh it accurately, put it in a 25ml measuring bottle, add mobile phase to dissolve and dilute to the mark, shake well, and use it as the reference substance solution.
[0103] Preparation of the test solution
[0104] Get about 1g of chloral hydrate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com