GLP-1 analogue-Fc fusion protein and preparation method and application thereof
A GLP-1 and fusion protein technology, applied in the biological field, can solve problems such as short half-life, achieve the effects of alleviating pain, improving affinity, and reducing the frequency of injections
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
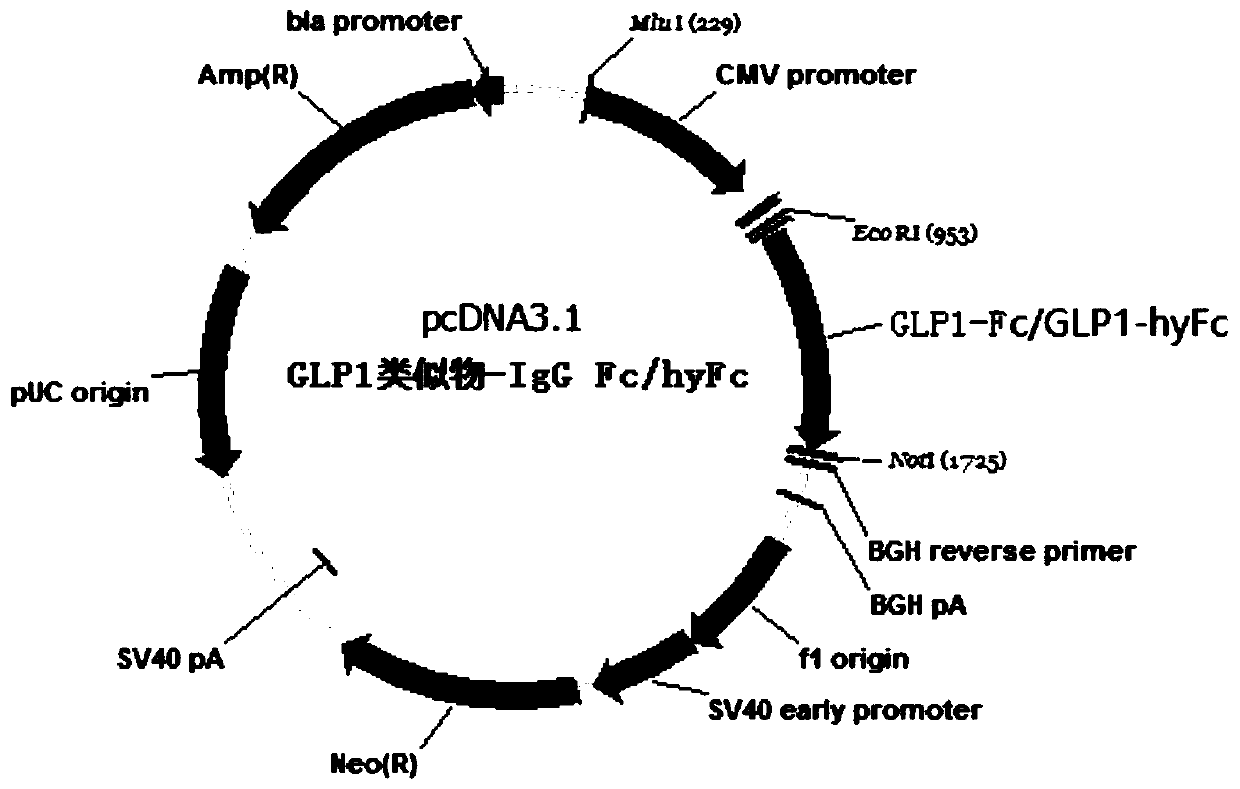
[0088] Embodiment 1 Construction of recombinant fusion protein expression vector
[0089] Using gene synthesis technology, the sequences of GLP-1 analogs, Fc, and connecting peptides are translated into DNA sequences according to the codon preference of animal cells for full sequence synthesis.
[0090] The nucleotide coding sequence of the GLP-1 analog is shown in SEQ ID NO.21, specifically:
[0091] CATGGCGAGGGCACCTTCACCTCCGACGTGTCTCTCCTATCTGGAGGAGCAGGCCGCCAAGGAGTTCATCGCCTGGCTGGTGAAGGGCGGCGGC.
[0092] 1. The nucleotide coding sequence of the GLP-1 analogue is fused with the nucleotide coding sequence of the connecting peptide and the nucleotide coding sequence of IgG-Fc to obtain the nucleoside of the GLP-1 analogue-IgG-Fc fusion protein Acid coding sequence:
[0093] Specifically, wherein, the nucleotide coding sequence of the connecting peptide is shown in SEQ ID NO.22, specifically:
[0094] GGTGGTGGTGGCTCCGGAGGCGGCGGCTCT.
[0095] The nucleotide coding sequence of I...
Embodiment 2
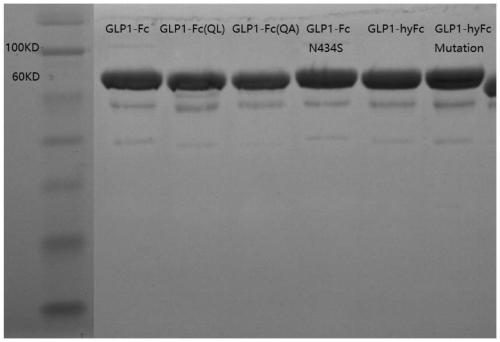
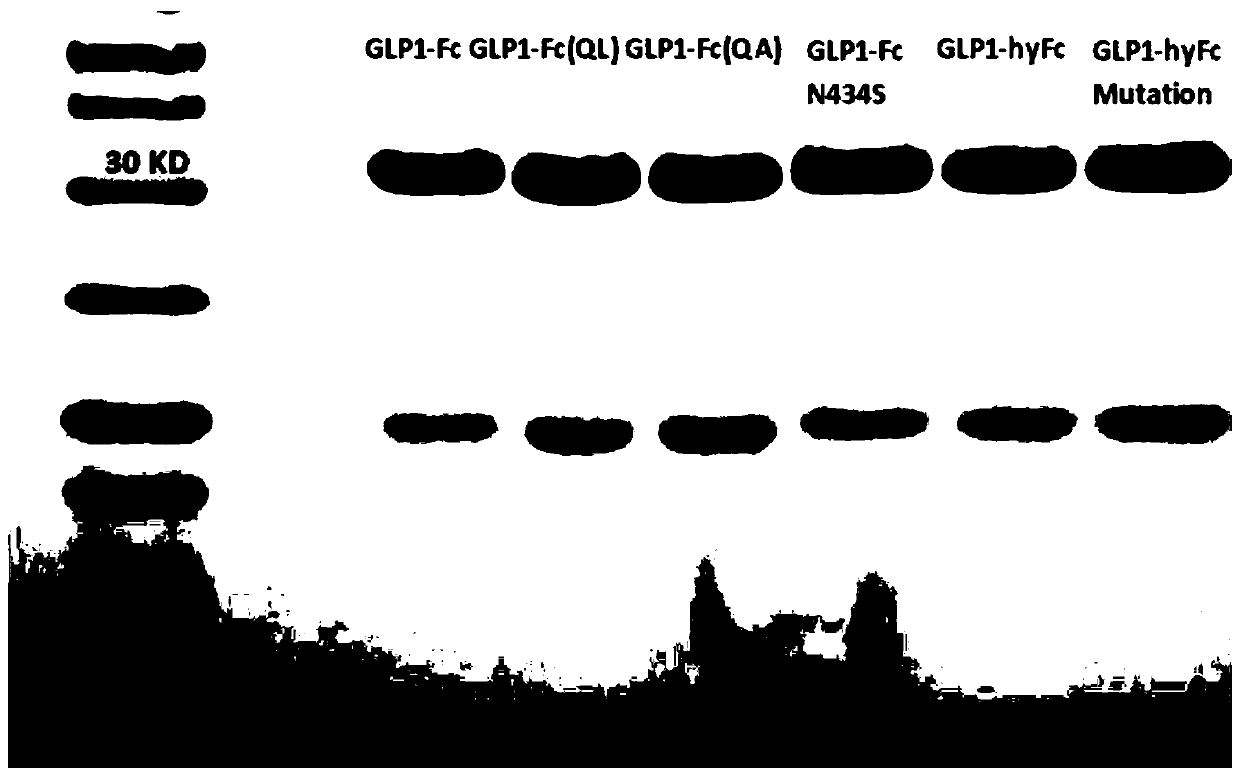
[0131] Embodiment 2 Expression and purification of recombinant fusion protein
[0132] 1. CHO expression of recombinant fusion protein
[0133] The recombinant fusion protein expression plasmids constructed in Example 1 were respectively transfected into CHO-S cells. In order to establish CHO-S cells stably expressing each recombinant fusion protein, a 6-well cell culture plate (2.5×10 5 cells / well) were transfected with 4 μg of linearized expression plasmids for each recombinant fusion protein. After 24 hours of transfection, the cells were divided and cultured in DMEM medium containing G418 (500 μg / ml) to select those cells that had stably integrated the recombinant fusion protein expression plasmid into their genome. During the culture process, the culture medium was changed every 3 days until cell clones formed. Isolate monoclonal cells and expand into stable cell lines, and use the human GLP1 analogue kit to detect fusion proteins from the tissue culture supernatants o...
Embodiment 3
[0151] Example 3 Biological Activity and Pharmacokinetics of Recombinant Fusion Protein
[0152] 1. Research on biological activity of recombinant fusion protein
[0153] When the GLP-1R receptor protein expressed by recombinant genetically engineered GLP-1R / HEK293 cells has physiological activity, when it is stimulated by the agonist GLP-1 or its functional analogues, the physiological metabolic activities of GLP-1R / HEK293 cells Enhanced, manifested as an increase in intracellular cAMP content. Therefore, whether the GLP-1R receptor protein expressed by GLP-1R / HEK293 cells has physiological activity can be determined by measuring the change of cAMP content in GLP-1R / HEK293 cells after being stimulated by agonists such as GLP-1. The specific method is as follows: GLP-1R / HEK293 cells were cultured in a 96-well culture plate at 100 μL / well (30,000 cells / well) in DMEM medium at 37°C, 5% CO 2 Conditioned for 24 hours. The next day, the complete medium was removed, and the basal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com