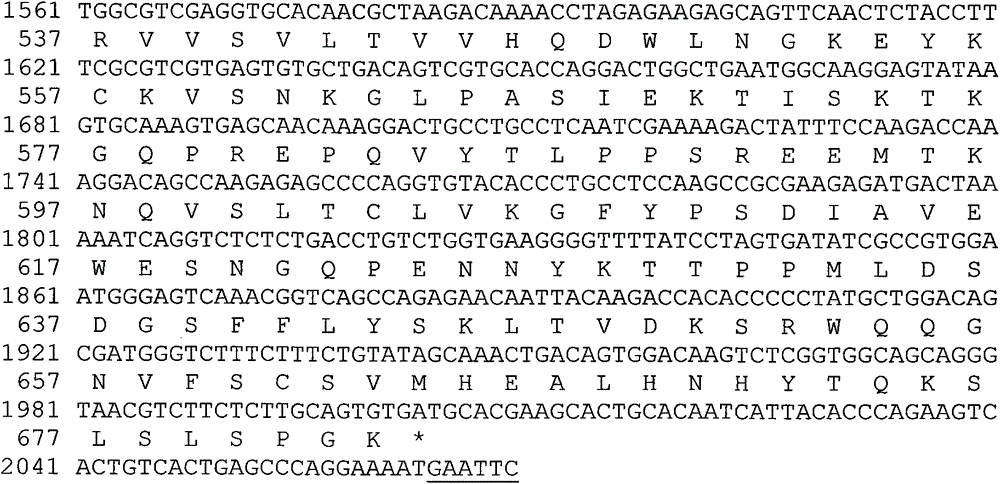Pharmaceutical composition containing modified type human coagulation factor FVII-Fc fusion protein
A technology of fusion protein and composition, applied in the field of treatment of various coagulation-related diseases, can solve the problems of no half-life FVII-Fc fusion protein, low expression amount, difficult construction, etc., to prolong the circulation half-life in vivo and reduce the frequency of injection , the effect of improving the quality of life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
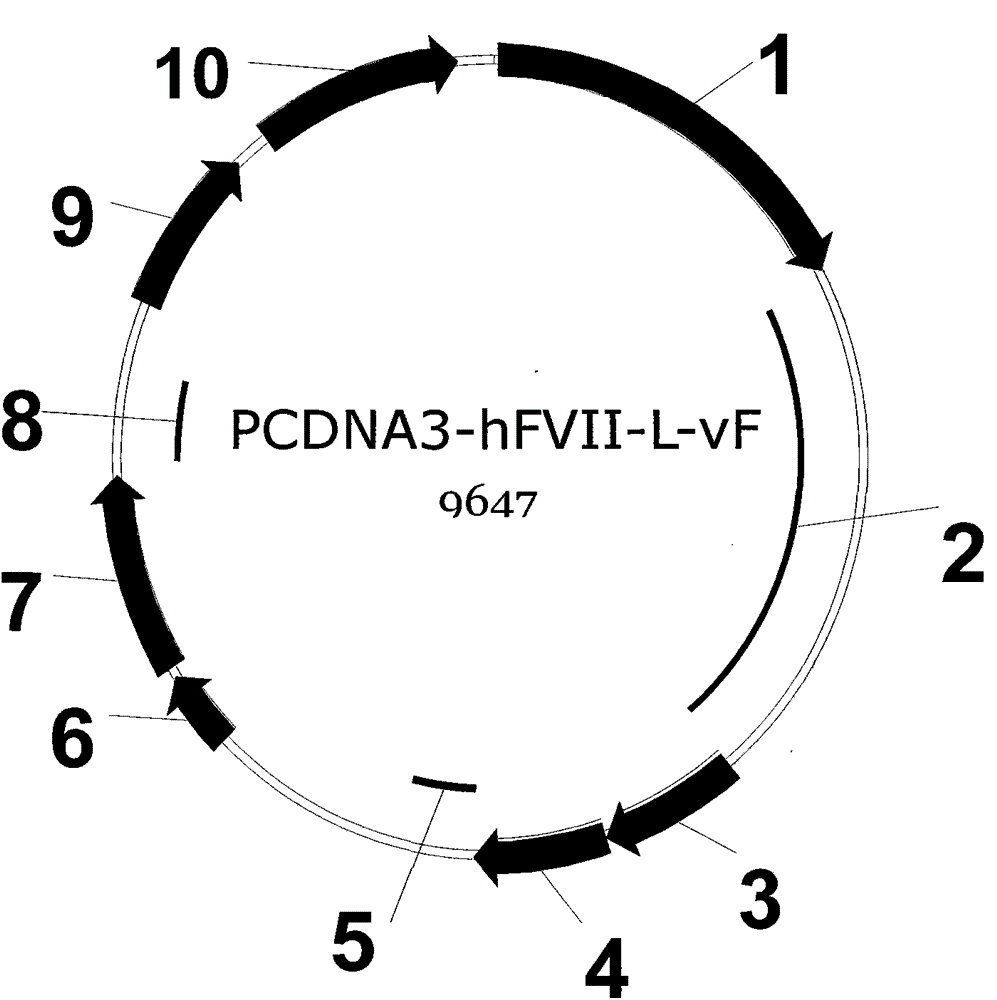
[0050] Example 1. Construction of an expression plasmid encoding hFVII-L-vFc fusion protein
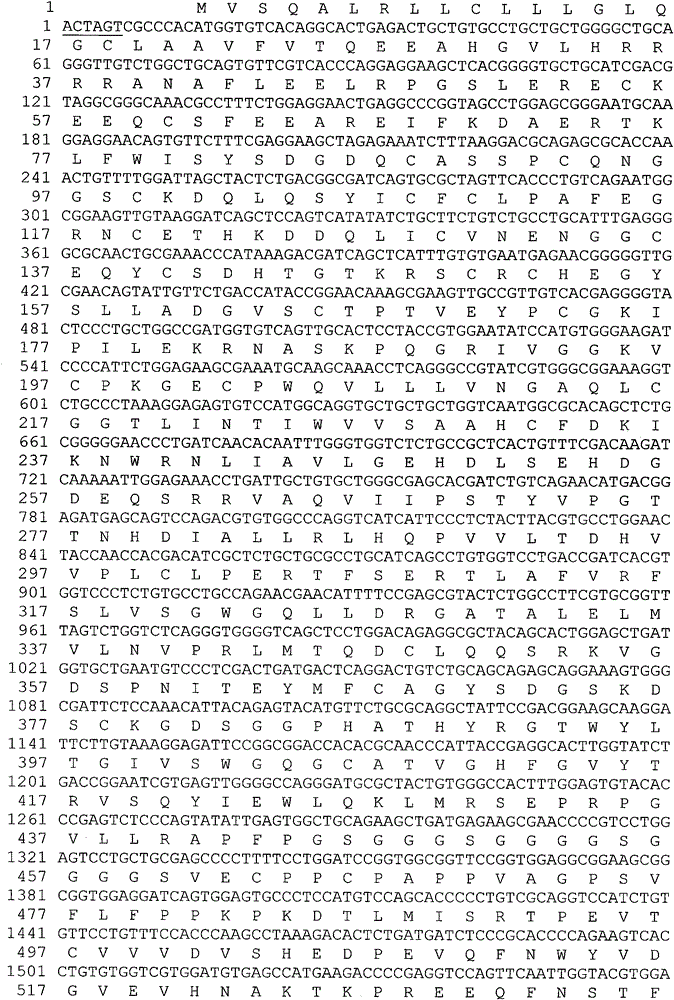
[0051] The target gene sequence encoding hFVII leader peptide and mature protein is artificially optimized CHO cell preferred codons and obtained by artificial synthesis. In order to facilitate the insertion of the target gene fragment into the specific site of the expression vector, there is a restriction enzyme endonuclease site at the 5' and 3' ends of the synthesized fragment, respectively SpeI and BamHI. The full-length 1351bp DNA fragment was inserted into the EcoRV restriction site of the transfer vector such as pUC57 to obtain an intermediate plasmid whose hFVII gene sequence was verified by DNA sequencing. The preferred fusion gene of the flexible peptide linker GlySer containing 2 amino acids in the present invention and the human IgG2vFc variant (Pro331Ser mutation) is also an artificially optimized codon preferred by CHO cells, and the 5' and 3' ends of the synthesized fra...
Embodiment 2
[0053] Example 2. Transient expression of fusion proteins and activity assays of flexible peptide linkers of different lengths
[0054] 5 kinds of expression plasmids that embodiment 1 obtains use DNAFect LT reagent (ATGCell) to transfect 3×10 in the shaking flask of 30ml 7 CHO-K1 cells, the transfected cells were grown for 5 days in the serum-free growth medium containing 100ng / ml vitamin K1, and the fusion protein concentration in the supernatant was measured by the method detailed in Example 8, and the The activity was determined by the method described in 7. ELISA results showed that the transient expression levels of FVII of the five plasmids were similar under this condition, but their coagulation activities showed great differences. The activity of the FVII supernatant expressed by the PFVII-A plasmid containing 2 amino acid linkers was the lowest, and the activity of the FVII supernatant expressed by the PFVII-B, PFVII-C, PFVII-D and PFVII-E plasmids was 113% of that ...
Embodiment 3
[0055] Example 3. Screening for stable transfected cell lines expressing fusion proteins
[0056] The above expression plasmid of PFVII-D (containing the peptide linker with the sequence GlySerGlyGlyGlyGlySerGlyGlyGlyGlyGlySerGlyGlyGlyGlyGlySer) was transfected into a mammalian host cell line to express hFVII-L-vFc fusion protein. In order to maintain stable high-level expression, the preferred host cells are DHFR-deficient CHO cells (US Patent No. 4,818,679). A preferred method of transfection is electroporation, although other methods including calcium phosphate co-sedimentation, lipofection, and microinjection can also be used. The electroporation method uses a Gene Pulser Electroporator (Bio-Rad Laboratories) set at a voltage of 250V and a capacitance of 1050μFd, and places 2 to 3×10 cells in a cuvette. 7 20 μg PvuI linearized expression plasmid was added to each cell, and the electroporated cells were transferred to a shake flask containing 30 ml growth medium. Two days...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com