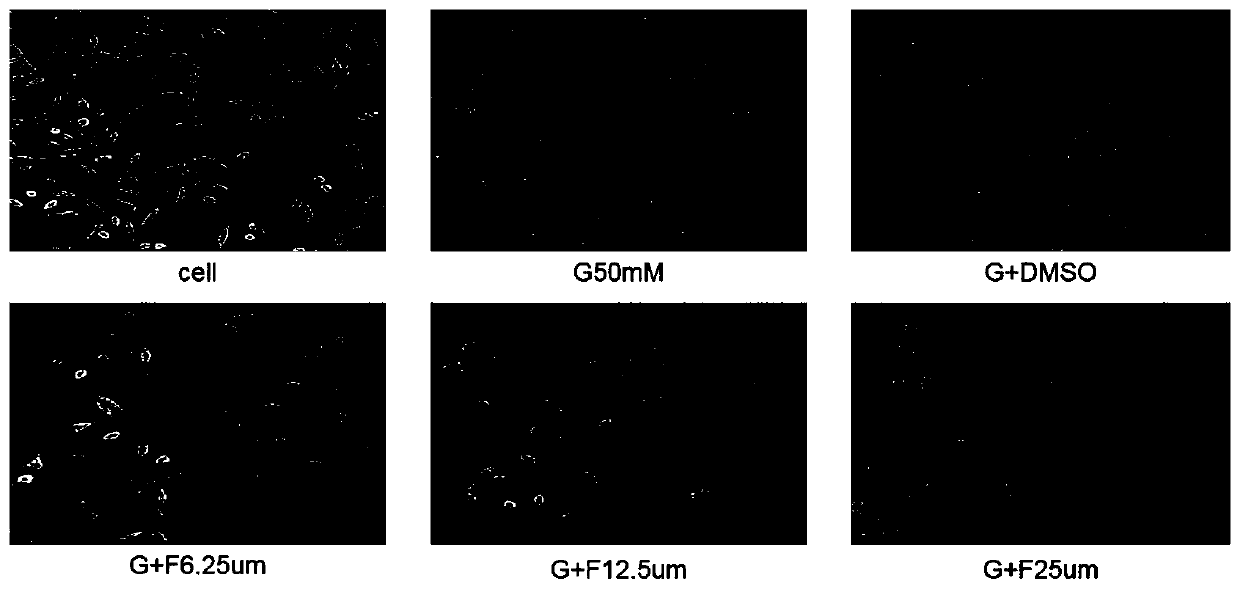
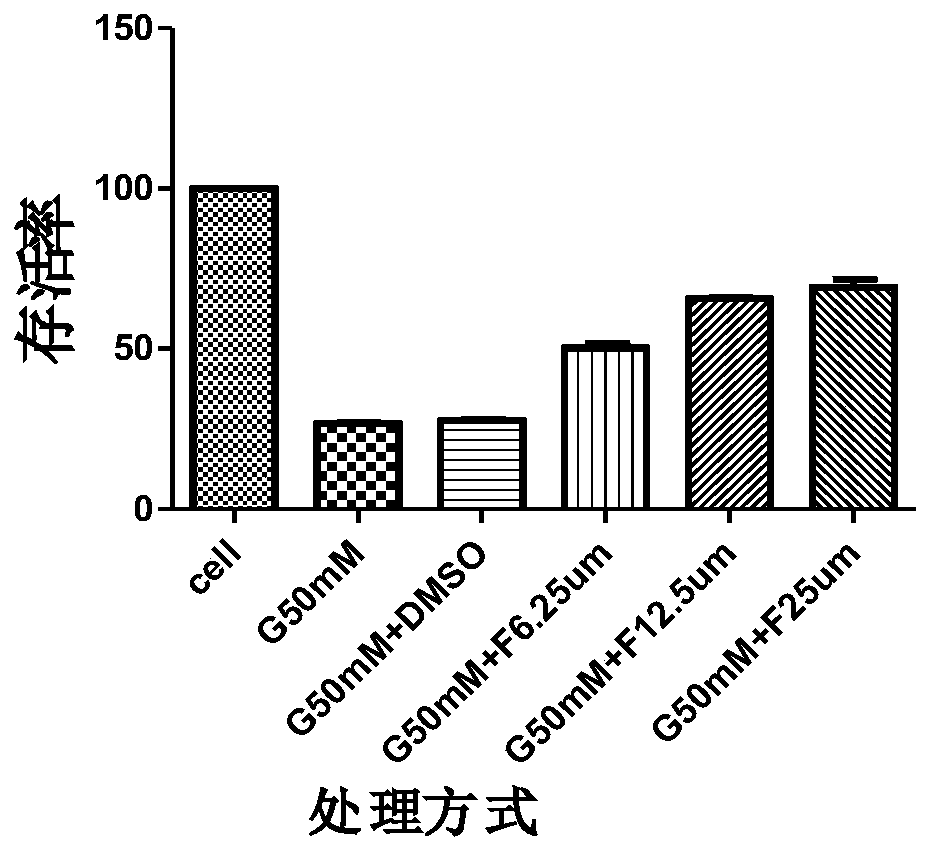
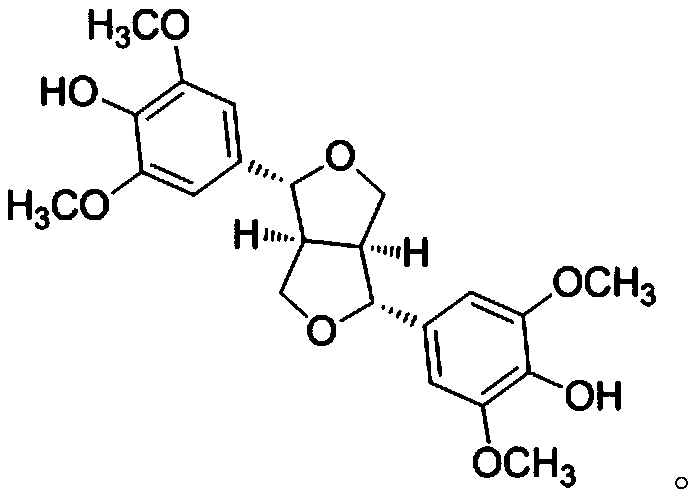
Application of syringaresinol in preparation of medicines for treating depression
A technology for syringaresinol and depression, which can be used in drug combinations, active ingredients of heterocyclic compounds, nervous system diseases, etc., and can solve problems such as difficulty in promotion, toxic side effects, and influence on daily life.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0016] The present invention will be further described below in conjunction with the examples, but not as the basis for limiting the present invention.
[0017] Embodiment of the invention
[0018] The specific steps of the antidepressant activity screening scheme of syringaresin mice in the present invention are as follows:
[0019] 1. Grouping and administration of animals
[0020] Kunming mice, 3-4 weeks old, male and female, weighing 20g-25g. The mice were completely randomly divided into 8 groups, 6 groups in total: blank group (no drug given), model group (distilled water), positive control group (imipramine hydrochloride, 15mg / kg), high-dose syringaresin group (10mg / kg), middle dose group (6mg / kg) and low dose group (2mg / kg). Imipramine hydrochloride and syringaresin were suspended and dispersed with 1% CMC-Na aqueous solution respectively. Each mouse (20 g) was orally administered with 0.2 ml, and all animals were orally administered before 10 am every day, once a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com