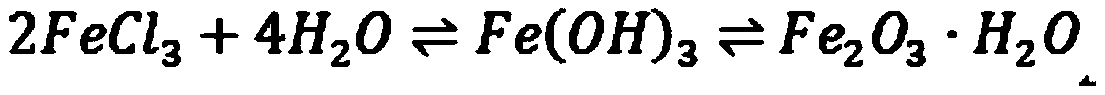Iron oxide yellow and preparation method thereof, iron oxide red and preparation method of iron oxide red
A technology of iron oxide yellow and iron oxide red, applied in the direction of iron oxide/iron hydroxide, iron oxide, etc., can solve the problem that the production rate and quality of iron oxide red cannot be balanced.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] A kind of preparation method of iron oxide yellow provided by the invention is specifically:
[0024] Firstly, the ferric salt solution and the alkaline precipitant used in the preparation process are prepared. The ferric salt solution is preferably a commonly used ferric chloride solution. Prepare two parts of ferric chloride solution in sufficient amount, the concentration of the first part of ferric chloride solution is 0.1-0.5 mol / L, and the concentration of the second part of ferric chloride solution is 1-2 mol / L. Prepare a sufficient amount of alkaline precipitant, divide the alkaline precipitant into the first alkaline precipitant and the second alkaline precipitant, the amount of the first alkaline precipitant is to completely precipitate the first ferric salt 1%-10% of the amount of the alkaline precipitating agent required by the solution, and the remaining alkaline precipitating agent is the second part of the alkaline precipitating agent. For avoiding that...
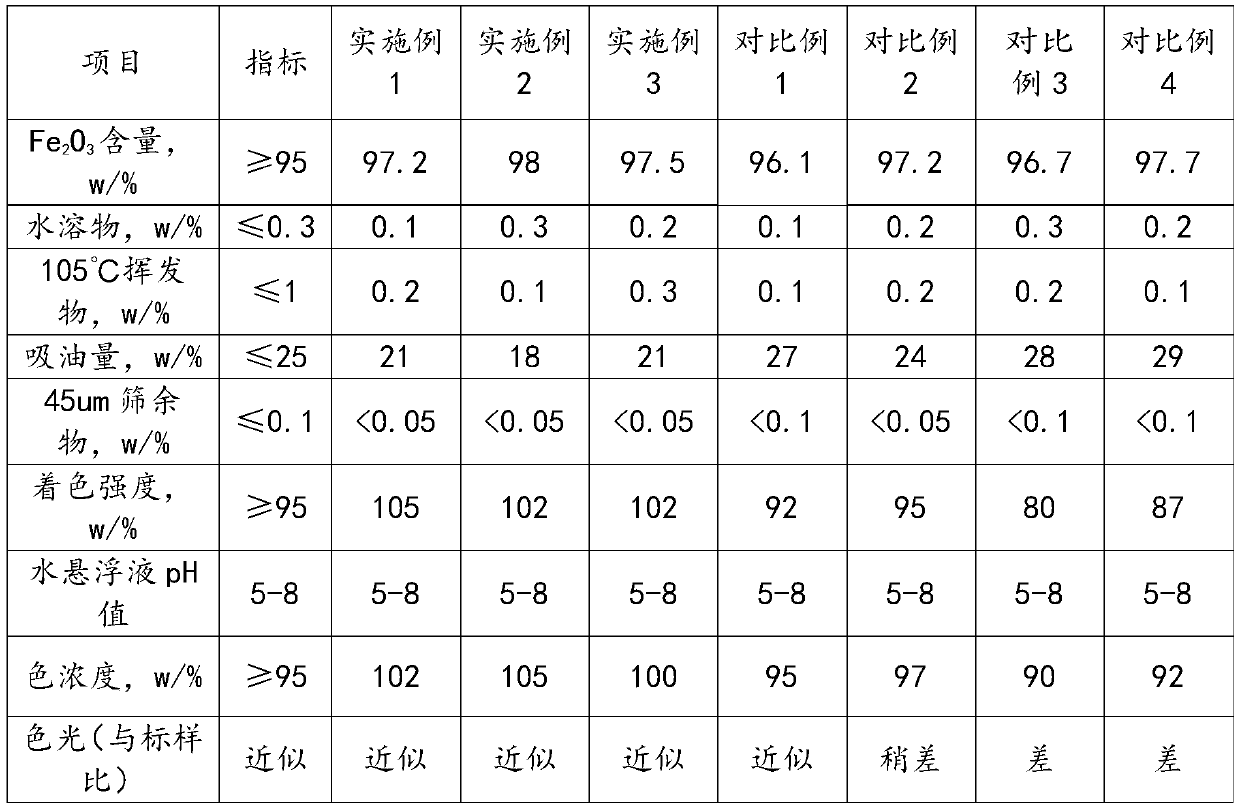
Embodiment 1
[0055] A kind of preparation method of iron oxide yellow that present embodiment provides, comprises:
[0056] 1000mL of 0.25mol / L FeCl 3 Solution, put 0.0075mol of the first part of ammonia water with a concentration of 5mol / L into the reactor, and raise the temperature to 90°C; add the second part of 5mol / L ammonia water into the reactor at a speed of 1mL / min, and stop adding ammonia water when the pH=1 ;Reduce the temperature of the reactor to 80°C and add 3.75×10 -4 mol of Na 2 HPO 4 , and then dropwise add FeCl with a concentration of 1mol / L to the reactor 3 solution, the rate of addition is 1mL / min, and the pH of the control reaction solution is within 1.4-1.6. The pH regulator used to control the reaction pH is ammonia water. The ammonia water used here is diluted with a volume ratio of 1:1. The mass concentration is 25% ammonia water; after reacting to the desired iron oxide yellow color, the iron oxide yellow provided by this embodiment is obtained. The preparati...
Embodiment 2
[0059] A kind of preparation method of iron oxide yellow that present embodiment provides, comprises:
[0060] 1000mL of 0.1mol / L FeCl 3 solution, 0.03mol concentration of 10mol / L of the first part of ammonia water was put into the reactor, and the temperature was raised to 85°C; the second part of 1 ammonia solution of concentration of 10mol / L was added into the reactor at a speed of 0.5mL / min, when the pH = Stop adding ammonia water at 1.5; lower the temperature of the reactor to 75°C and add 3×10 -4 mol of K 2 SiO 3 , and then dropwise add FeCl with a concentration of 2mol / L to the reactor 3 solution, the rate of addition is 1mL / min, and the pH of the control reaction solution is within 1.8-2. The pH regulator used to control the pH of the reaction is ammonia water. The ammonia water used here is diluted with a volume ratio of 1:1. The mass concentration is 28% ammonia water; after reacting to the desired iron oxide yellow color, the iron oxide yellow provided by this e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com