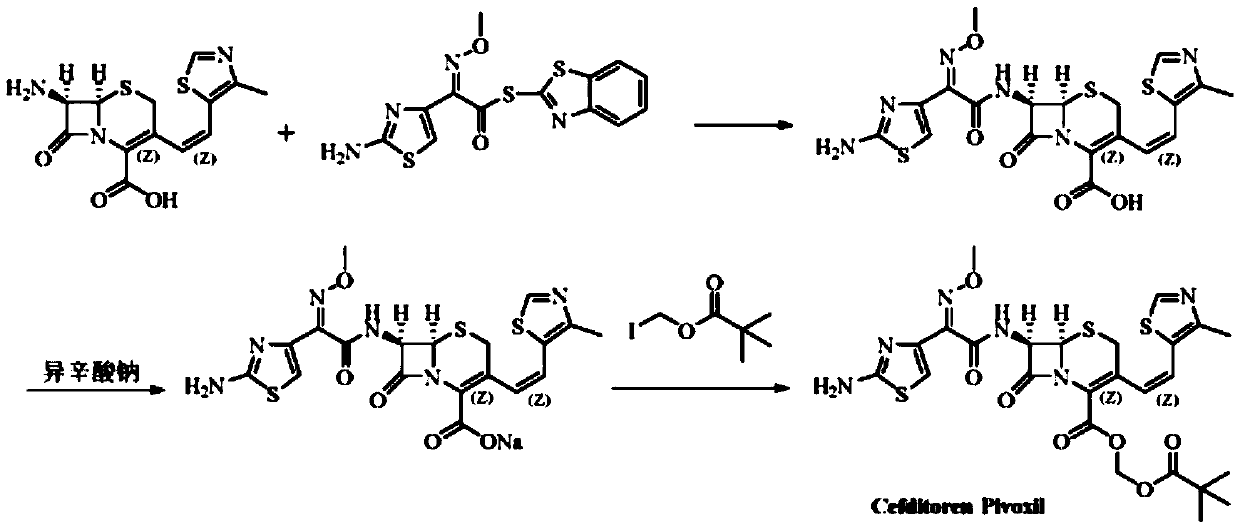
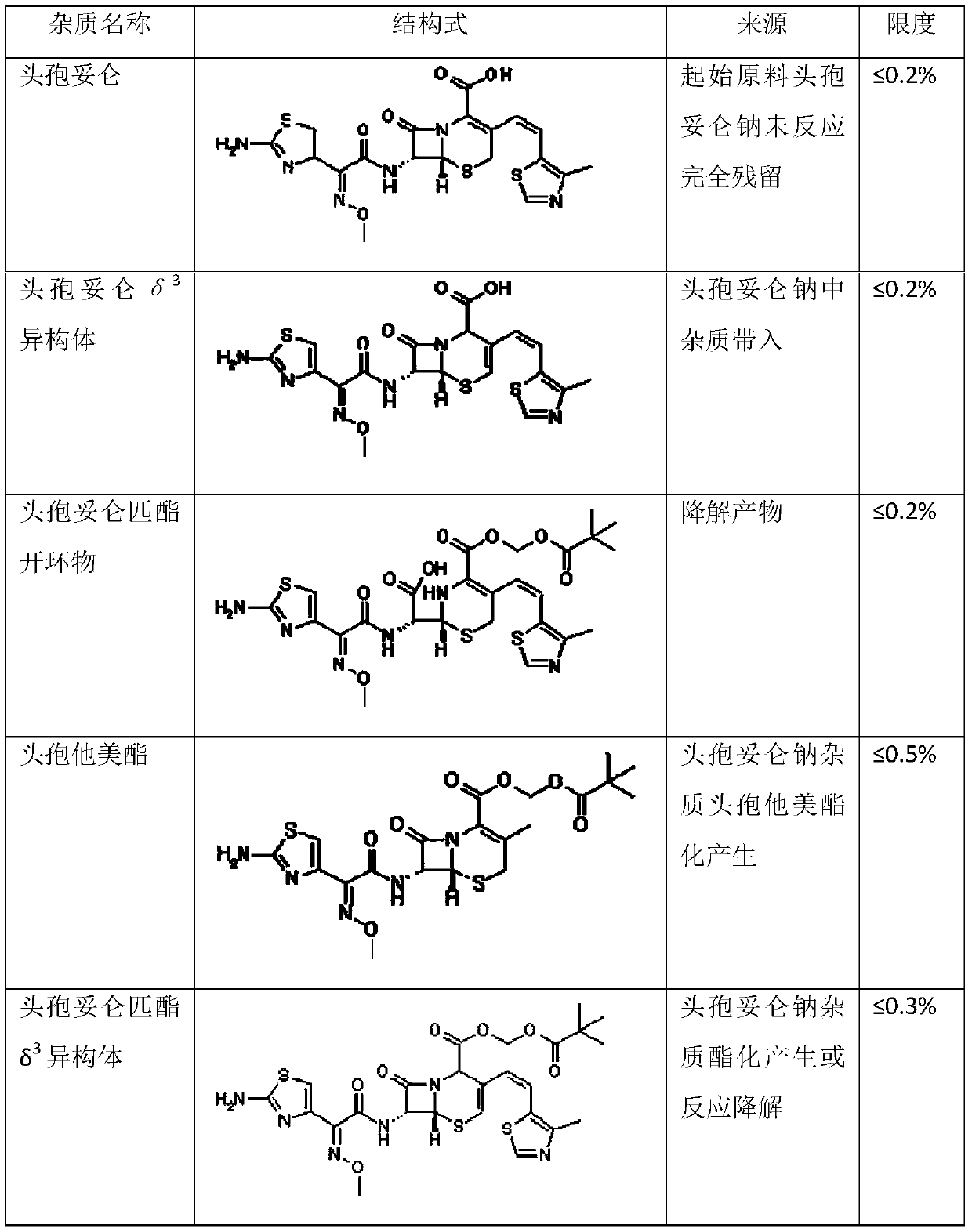
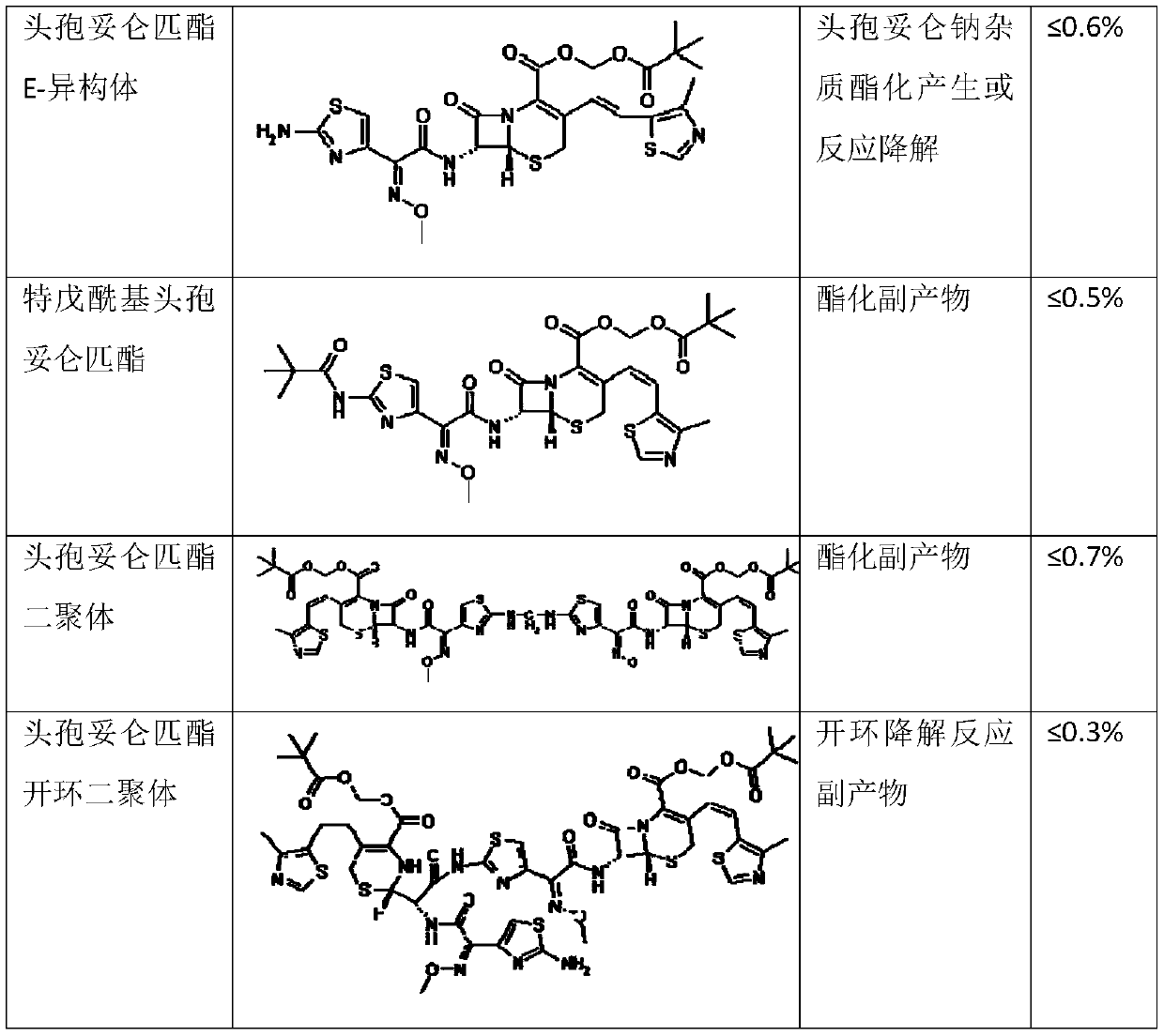
Method for preparing cefditoren pivoxil open-cycle dimer
A technology of cefditoren pivoxil and fiditoren pivoxil, applied in the field of impurity analysis in drug synthesis, can solve the problems of affecting accuracy, difficult to completely separate impurities, cumbersome steps, etc., and achieve low cost and safe clinical use Guaranteed, easy-to-step results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The preparation of the cefditoren pivoxil protected by A, Cbz
[0037] Add 6.2g (0.01mol) of cefditoren pivoxil and 100ml of dichloromethane into the reaction flask, stir and cool down to 0°C, add 0.0101mol of benzyl chloroformate dropwise, and simultaneously add triethylamine dropwise to control the pH of the system to 6.5 After the reaction, add 100ml of water, stir for 15 minutes, let stand to separate layers, wash the organic layer with water, concentrate under reduced pressure to obtain an oil, and proceed to the next step without purification.
[0038] Preparation of the cefditoren pivoxil ring-opened product of B, Cbz protection
[0039] Dissolve the oil from the previous step in 50ml of dimethyl sulfoxide, stir and cool down to -20°C, slowly add 0.01mol sodium hydroxide, heat up to 10°C for reaction, after HPLC checks that the reaction is complete, add 50ml of water and 50ml of ethyl acetate , adjust the pH value to 3.5 with 5% hydrochloric acid under stirring,...
Embodiment 2
[0055] The preparation of the cefditoren pivoxil protected by A, Cbz
[0056] Add 6.2g (0.01mol) of cefditoren pivoxil and 100ml of chloroform into the reaction flask, stir and cool down to 5°C, add 0.012mol of benzyl chloroformate dropwise, and at the same time add diethylamine dropwise to control the pH of the system to 7.5 After the addition, react at about 15°C. After the reaction, add 100ml of water, stir for 15 minutes, let stand to separate layers, wash the organic layer with water, concentrate under reduced pressure to obtain an oil, and directly proceed to the next step without purification.
[0057] Preparation of the cefditoren pivoxil ring-opened product of B, Cbz protection
[0058] Dissolve the oil from the previous step in 50ml of N,N-dimethylformamide, stir and cool down to -15°C, slowly add 0.012mol potassium hydroxide, raise the temperature to 20°C for reaction, after HPLC checks that the reaction is complete, add 50ml of water and 50ml of ethyl acetate, adj...
Embodiment 3
[0065] The preparation of the cefditoren pivoxil protected by A, Cbz
[0066] Add 6.2g (0.01mol) of cefditoren pivoxil and 100ml of dichloromethane into the reaction flask, stir and lower the temperature to 2°C, add 0.0105mol of benzyl chloroformate dropwise, and simultaneously add diisopropylethylamine dropwise to control the system The pH value is 7.0, react at about 10°C after the addition, after the reaction, add 100ml of water, stir for 15 minutes, let stand to separate layers, wash the organic layer with water, concentrate under reduced pressure to obtain an oily substance, and proceed to the next step without purification .
[0067] Preparation of the cefditoren pivoxil ring-opened product of B, Cbz protection
[0068] Dissolve the oil from the previous step in 50ml of N-methylpyrrolidone, stir and cool down to -10°C, slowly add 0.0105mol potassium carbonate, heat up to 15°C for reaction, after HPLC checks that the reaction is complete, add 50ml of water and 50ml of et...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com