Kit and method for HPV (human papillomavirus) parting detection
A technology of kits and molecular beacons, applied in biochemical equipment and methods, and microbial determination/inspection, etc., can solve the problems of low sensitivity, high cost, and poor specificity of HPV typing, and achieve high sensitivity, low requirements, and specificity strong effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1 is used for the kit and method of HPV typing detection
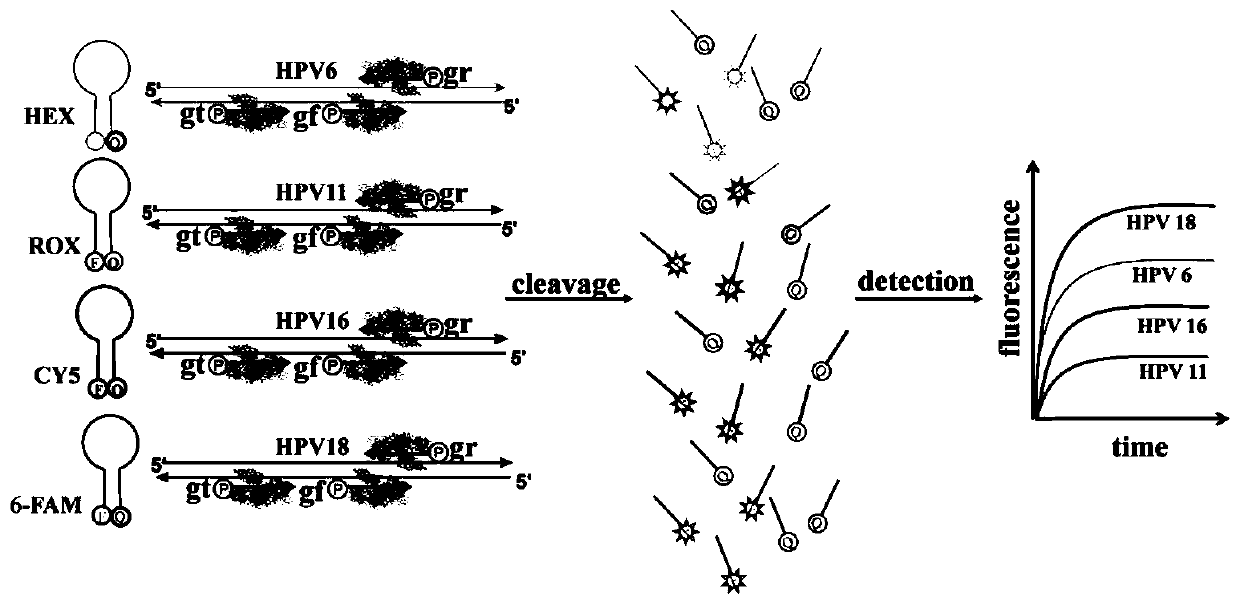
[0039] 1. Quadruple PCR amplification to obtain PCR amplification products
[0040] 1. Design of PCR amplification primers
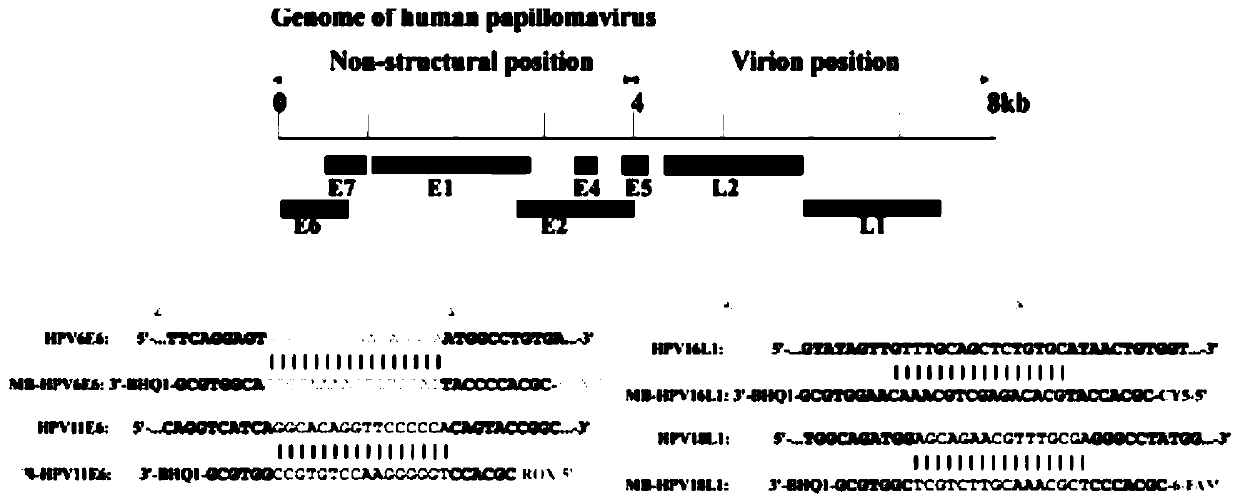
[0041] The upstream and downstream primer sets of the four HPVs are mainly designed for the E6 genes of HPV6 and HPV11 and the L1 genes of HPV16 and HPV18, so that the corresponding DNA fragments can be respectively amplified in the same PCR system.
[0042] The upstream and downstream primer sets of HPV6: the upstream primers of HPV6 are shown in SEQ ID NO.1, and the downstream primers of HPV6 are shown in SEQ ID NO.2;
[0043] The upstream and downstream primer sets of HPV11: the upstream primer of HPV11 is shown in SEQ ID NO.3, and the downstream primer of HPV11 is shown in SEQ ID NO.4;
[0044] The upstream and downstream primer sets of HPV16: the upstream primers of HPV16 are shown in SEQ ID NO.5, and the downstream primers of HPV16 are shown in SEQ ID NO.6;
[0045] The u...
Embodiment 2
[0101] The detection of embodiment 2 positive standard substance
[0102] 1. Acquisition of positive standard products
[0103] Take the positive sample determined to contain at least one of HPV6, HPV11, HPV16, and HPV18 as a positive standard, and use Anbiping’s human papillomavirus nucleic acid detection kit to detect the sample. Positive samples (including high-risk human papilloma virus samples and low-risk human papillomavirus samples) as the positive standard of the present invention.
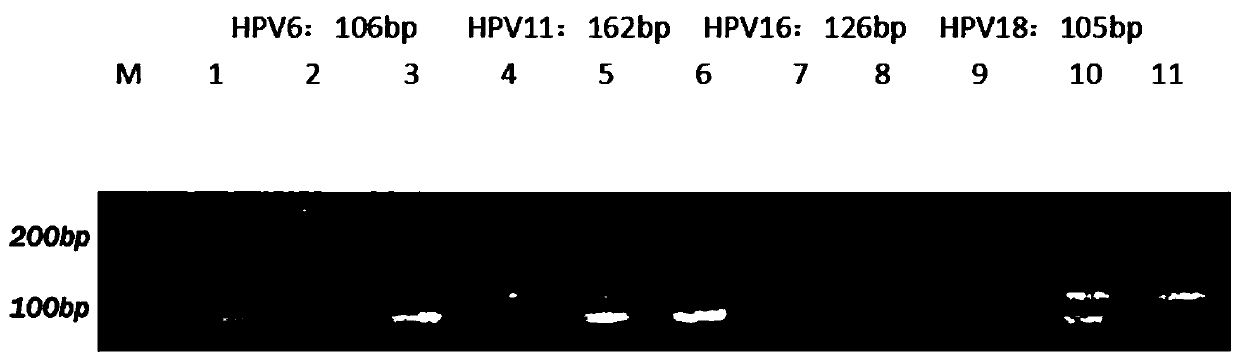
[0104] 2, quadruple PCR amplification obtains PCR amplification product: adopt the method for embodiment 1 to carry out quadruple PCR amplification to obtain amplification product after above-mentioned positive standard substance; image 3 It is the result figure of the agarose gel electrophoresis experiment of the amplified product obtained after performing quadruple PCR amplification on the positive standard provided by the example of the present invention, wherein, 1 swimming lane is ...
Embodiment 3
[0108] Embodiment 3 sensitivity analysis and specificity analysis
[0109] 1. Using the positive standard as a template, take the detection sensitivity analysis of HPV6 upstream and downstream primers and molecular beacon probes as an example.
[0110] 1. Acquisition of different gradient positive standards
[0111] Take 20 μL of HPV6 positive standard and add 180 μL of DEPC-treated water, and perform serial dilutions to obtain positive standards with different gradients. 10 respectively 9 aM, 10 8 aM, 10 7 aM, 10 6 aM, 10 5 aM, 10 4 aM, 10 3 aM, 10 2 aM, 10 1 aM, 10 0 aM, 10 -1 aM, 10 -2 aM, 10 -3 aM, 10 -4 aM;
[0112] 2, adopt the method for embodiment 1 to carry out quadruple PCR amplification to the positive standard substance of above-mentioned different concentrations and obtain the amplified product;
[0113] 3. The product obtained by PCR amplification is mixed with the above-mentioned DNA guide and molecular beacon, and then added with Pfago protease ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com