A kind of pretreatment liquid of exfoliated cells of rectal mucosa and its preparation method
A technology of pretreatment solution and exfoliated cells, which is applied in botany equipment and methods, preparation of test samples, chemicals for biological control, etc., can solve problems affecting the accuracy of test results, etc., and achieve elimination and inhibition The effect of breeding, ensuring accuracy, and maintaining integrity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
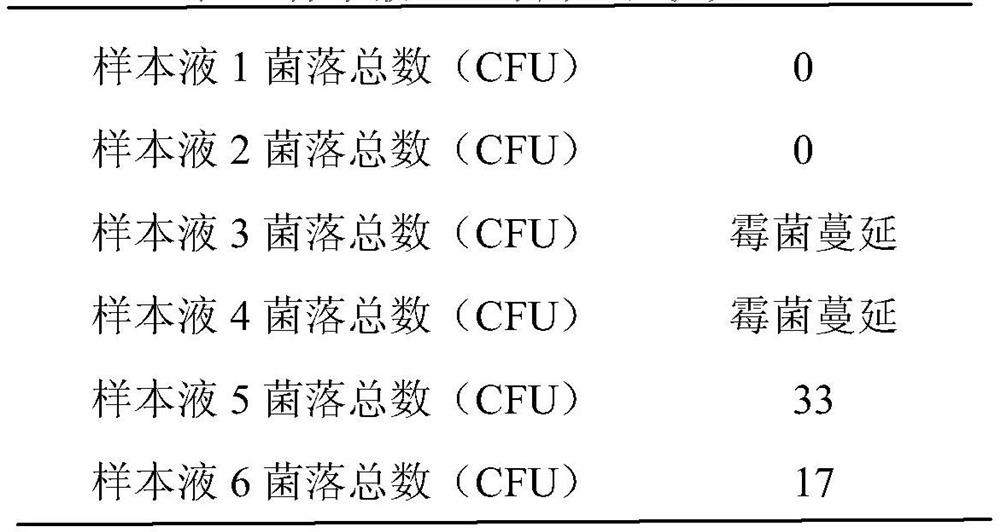
[0024] Embodiment 1 illustrates mold inhibition test
[0025] 1. Configure pretreatment liquids with different disinfectants
[0026] 1. Verification solution 1 (treatment solution before the present invention)
[0027] (1) Dissolve 9g of sodium chloride in 1000ml of distilled water;
[0028] (2) Dissolve 72.8 g of trishydroxymethylaminomethane, 9.3 g of disodium edetate, and 0.2 g of ethyl phenyl polyethylene glycol in the upper liquid;
[0029] (3) Dissolve 65 g of shell iodine and 0.1 ml of methylisothiazolinone in the above liquid and mix well.
[0030] 2. Verification solution 2 (only containing disinfectant)
[0031] (1) Dissolve 9g of sodium chloride in 1000ml of distilled water;
[0032] (2) Dissolve 65g of shell iodine and 0.1ml of methylisothiazolinone in the above liquid and mix well.
[0033] 3. Verification solution 3 (no disinfectant)
[0034] (1) Dissolve 9g of sodium chloride in 1000ml of distilled water;
[0035] (2) Dissolve 72.8 g of trishydroxymethyl...
Embodiment 2
[0062] Example 2 illustrates the observation of cell integrity
[0063] 1. Configure pretreatment fluids with different tensions
[0064] 1. Verification solution 1 (treatment solution before the present invention)
[0065] (1) Dissolve 9g of sodium chloride in 1000ml of distilled water;
[0066] (2) Dissolve 72.8 g of trishydroxymethylaminomethane, 9.3 g of disodium edetate, and 0.2 g of ethyl phenyl polyethylene glycol in the upper liquid;
[0067] (3) Dissolve 65 g of shell iodine and 0.1 ml of methylisothiazolinone in the above liquid and mix well.
[0068] 2. Verification solution 2 (low tension pretreatment solution)
[0069] (1) Take 1000ml of distilled water;
[0070] (2) Dissolve 72.8 g of trishydroxymethylaminomethane, 9.3 g of disodium edetate, and 0.2 g of ethyl phenyl polyethylene glycol in the upper liquid;
[0071] (3) Dissolve 65 g of shell iodine and 0.1 ml of methylisothiazolinone in the above liquid and mix well.
[0072] 3. Verification solution 3 (hi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com