A method for realizing complete debromination of polybrominated aromatic compounds by photoreduction
A technology of aromatic compounds and bromination, applied in the field of photochemical synthesis, can solve problems such as strong toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
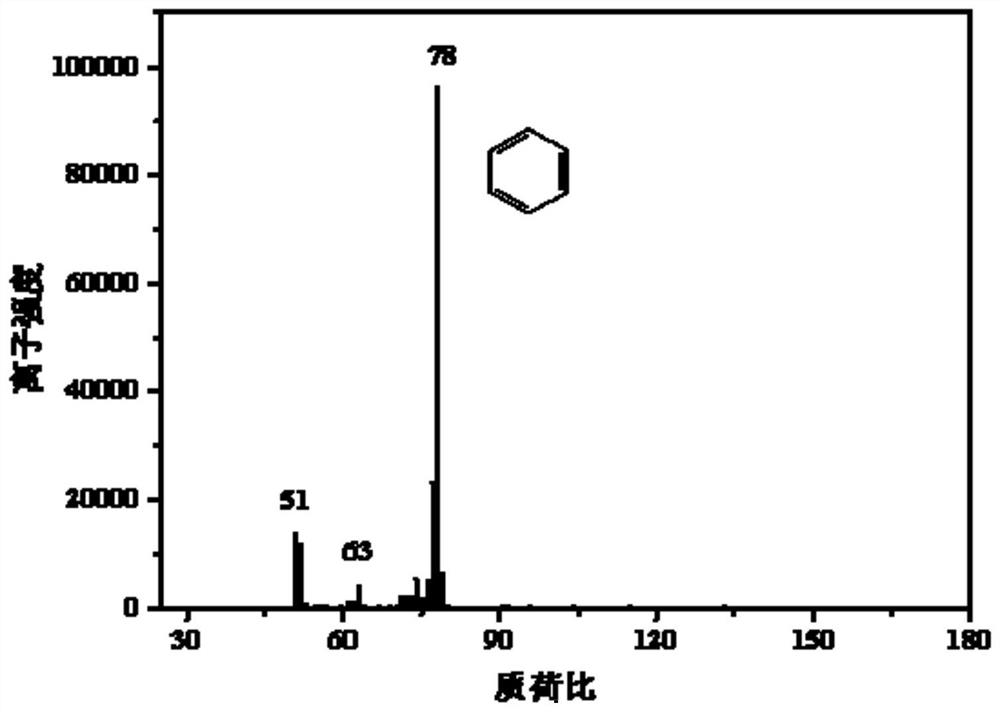
[0040] Add N,N-dimethylacetamide:hexabromobenzene in a molar ratio of 50:1 into the photochemical reactor filled with acetonitrile solvent. Then, the photoreactor was sealed, and the solution was ultrasonically mixed for 10 minutes, and the photochemical reactor was deoxygenated with high-purity argon, and stirred while deoxygenating. After deoxygenation was completed, the reaction was stopped after continuing to stir under the same conditions and irradiating with ultraviolet light for 2 hours. The main product of the reaction is benzene.
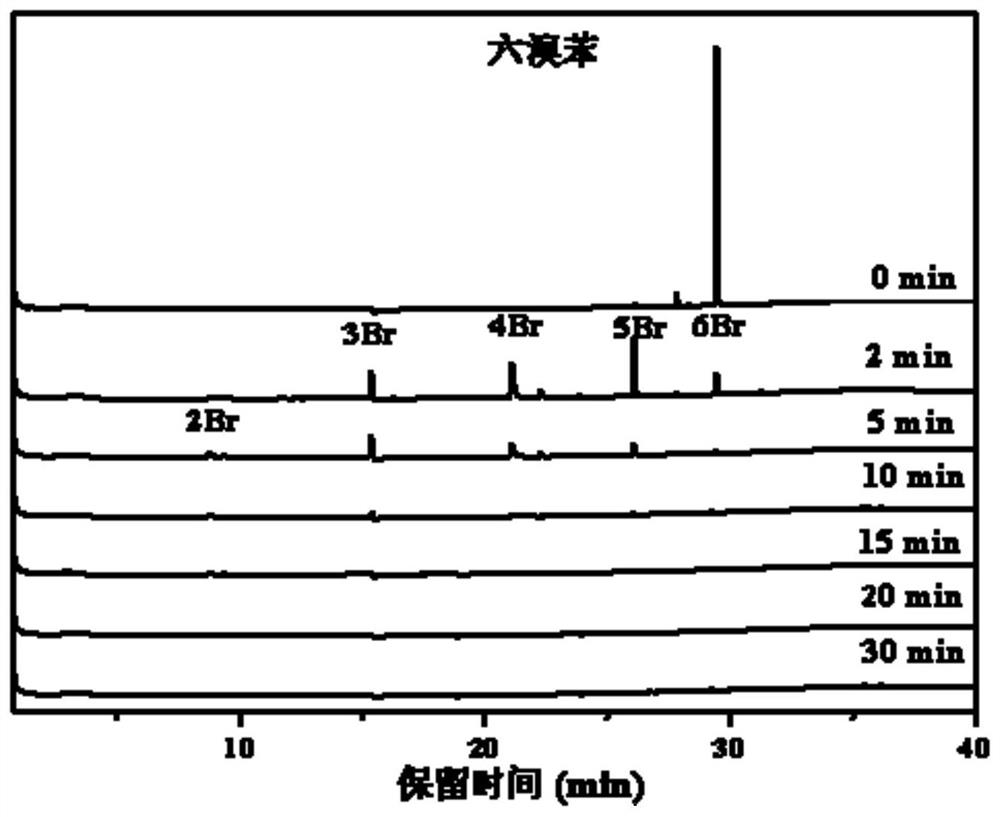
[0041] GC-μECD was used to detect the degradation products of hexabromobenzene (HBB) at different irradiation times, such as figure 1 As shown in the figure, it can be seen from the figure that with the illumination, a large amount of pentabromobenzene, tetrabromobenzene and tribromobenzene appeared in the gas spectrum detection in two minutes, and in five minutes, hexabromobenzene was completely transformed, and dibromobenzene appeared B...
Embodiment 2
[0044] Add N,N-dimethylaniline:decabromobiphenyl in a molar ratio of 50:1 into the photochemical reactor filled with acetonitrile solvent. Then, the photoreactor was sealed, and the solution was ultrasonically mixed for 10 minutes, and the photochemical reactor was deoxygenated with high-purity argon, and stirred while deoxygenating. After the deoxygenation is completed, continue to stir under the same conditions and stop the reaction after irradiating with ultraviolet light for 4 hours. The main product of the reaction is biphenyl.
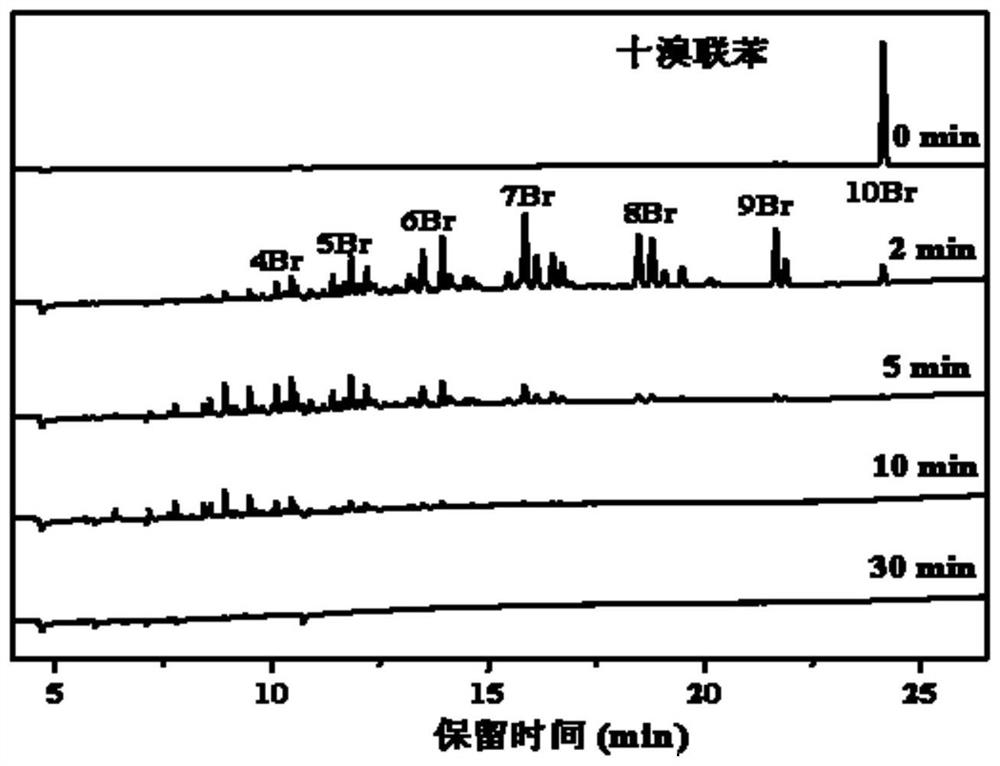
[0045] GC-μECD was used to detect the degradation products of decabromobiphenyl (DBB) at different irradiation times, such as image 3 As shown, it can be seen from the figure that as the light is carried out, in two minutes, decabromobiphenyl is converted in a large amount to generate a series of low brominated products (4-9 bromine). Continue to illuminate, and the low-brominated products continue to be converted. In five minutes, the decabro...
Embodiment 3
[0048] Add N,N,N',N'-tetramethyl-p-phenylenediamine:tetrabromodiphenyl ether in a molar ratio of 50:1 into a photochemical reactor filled with methanol solvent. Then, the photoreactor was sealed, and the solution was ultrasonically mixed for 10 minutes, and the photochemical reactor was deoxygenated with high-purity argon, and stirred while deoxygenating. After deoxygenation, continue to stir under the same conditions and stop the reaction after irradiating with visible light for 4 hours. The main product of the reaction is biphenyl. The conversion rate of the reactant tetrabromodiphenyl ether is 100%, and the yield of diphenyl ether is 48%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com