Preparation of flagellin vaccine adjuvant-based vaccine to induce production of antibody recognizing conformation of antigens, and application thereof
An antigen recognition and isomer technology, applied in the direction of antigen/adjuvant combination components, antiviral agents, bacterial antigen components, etc., can solve problems such as neuropathic damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0098] Embodiment 1: Preparation of Alzheimer's disease immune vaccine
[0099] Experimental Materials
[0100] Cultivation and storage of each strain
[0101] The Escherichia coli strain used in the present invention was cultured in LB (Luria Bertani) medium (Difco Co.). After culturing, the strain used was added to make the oil content 30%, and then stored in an ultra-low temperature freezer at -80°C.
[0102] The strains and plasmids used in the present invention are listed in Table 1.
[0103] Table 1
[0104]
[0105] Experimental method and experimental results
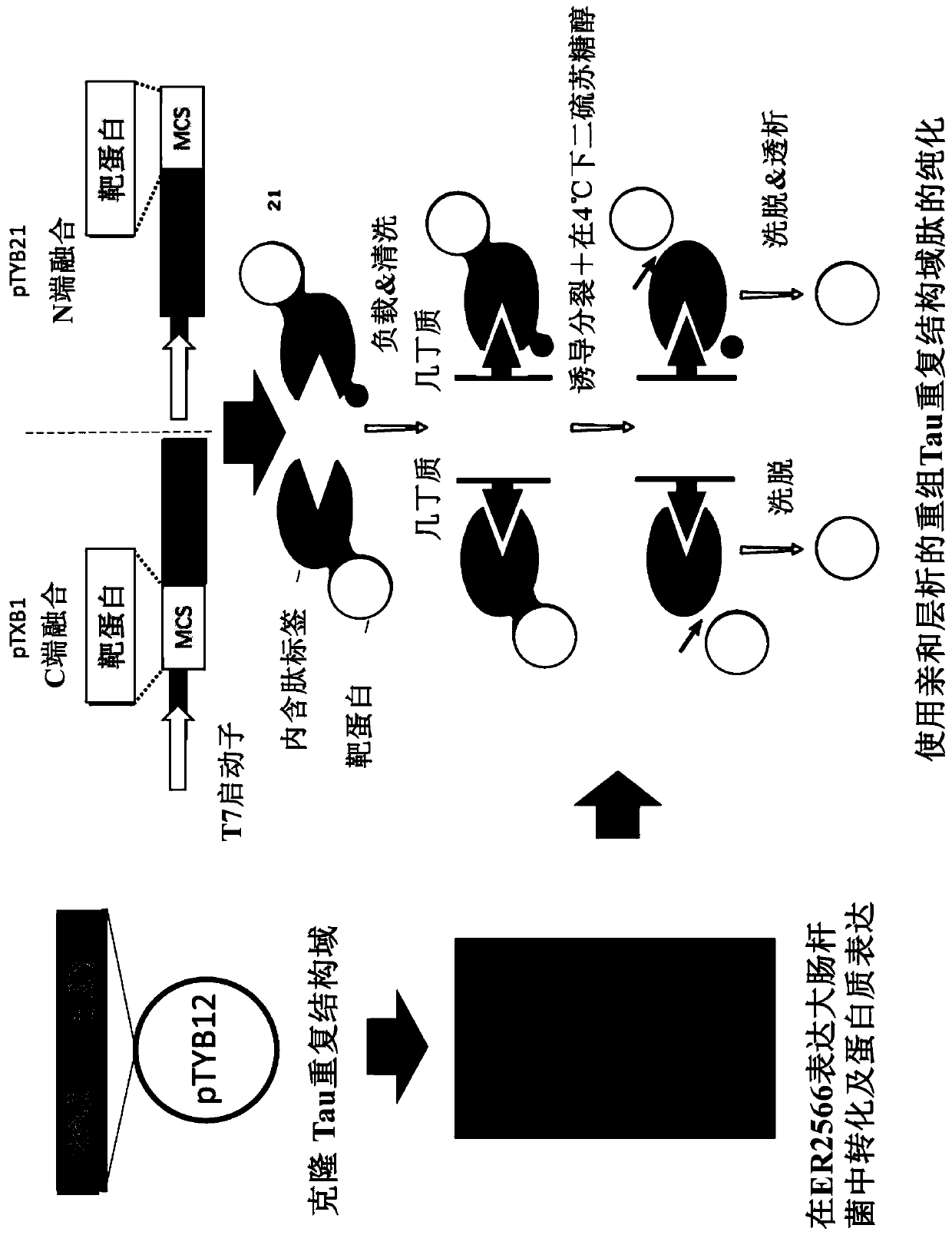
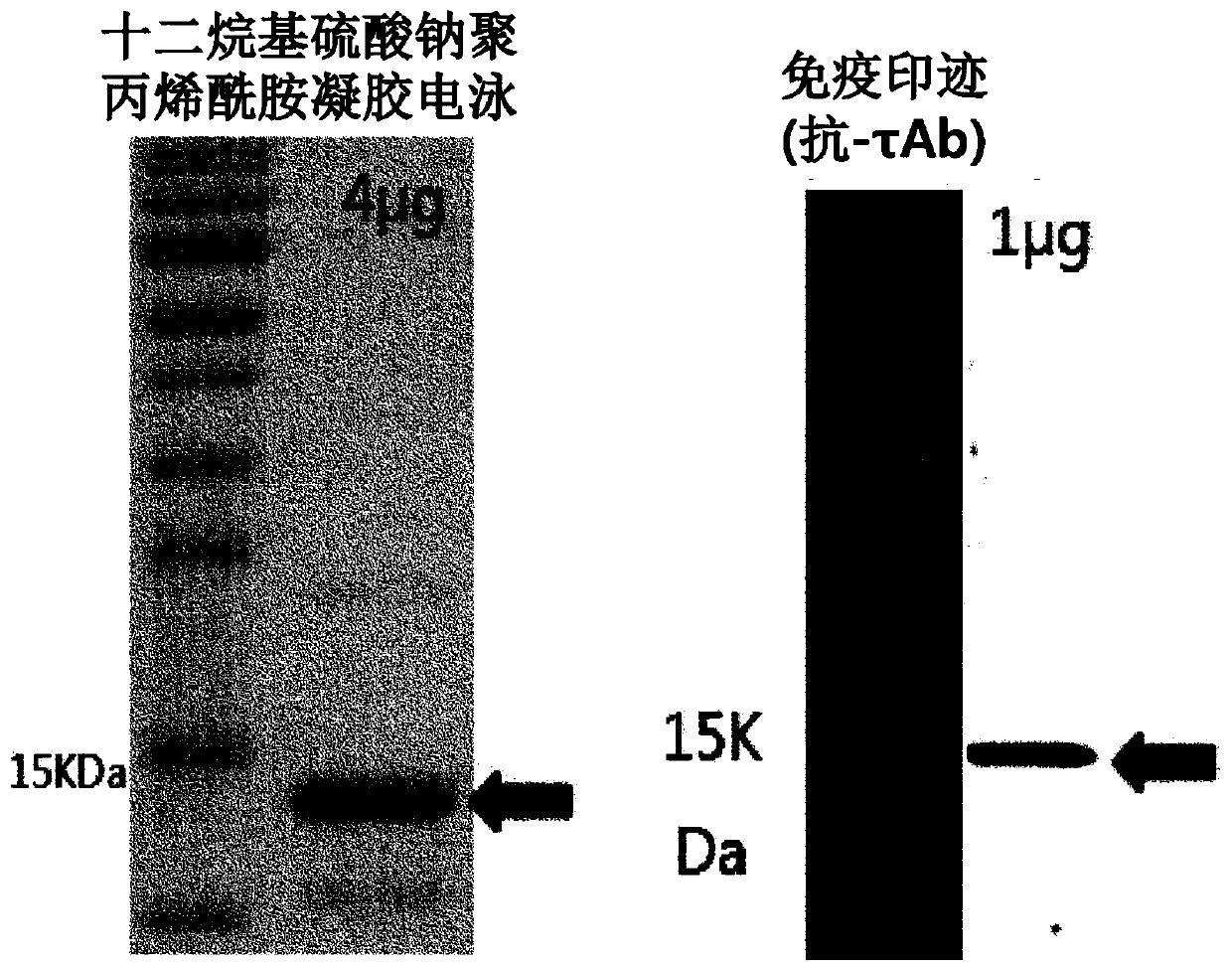
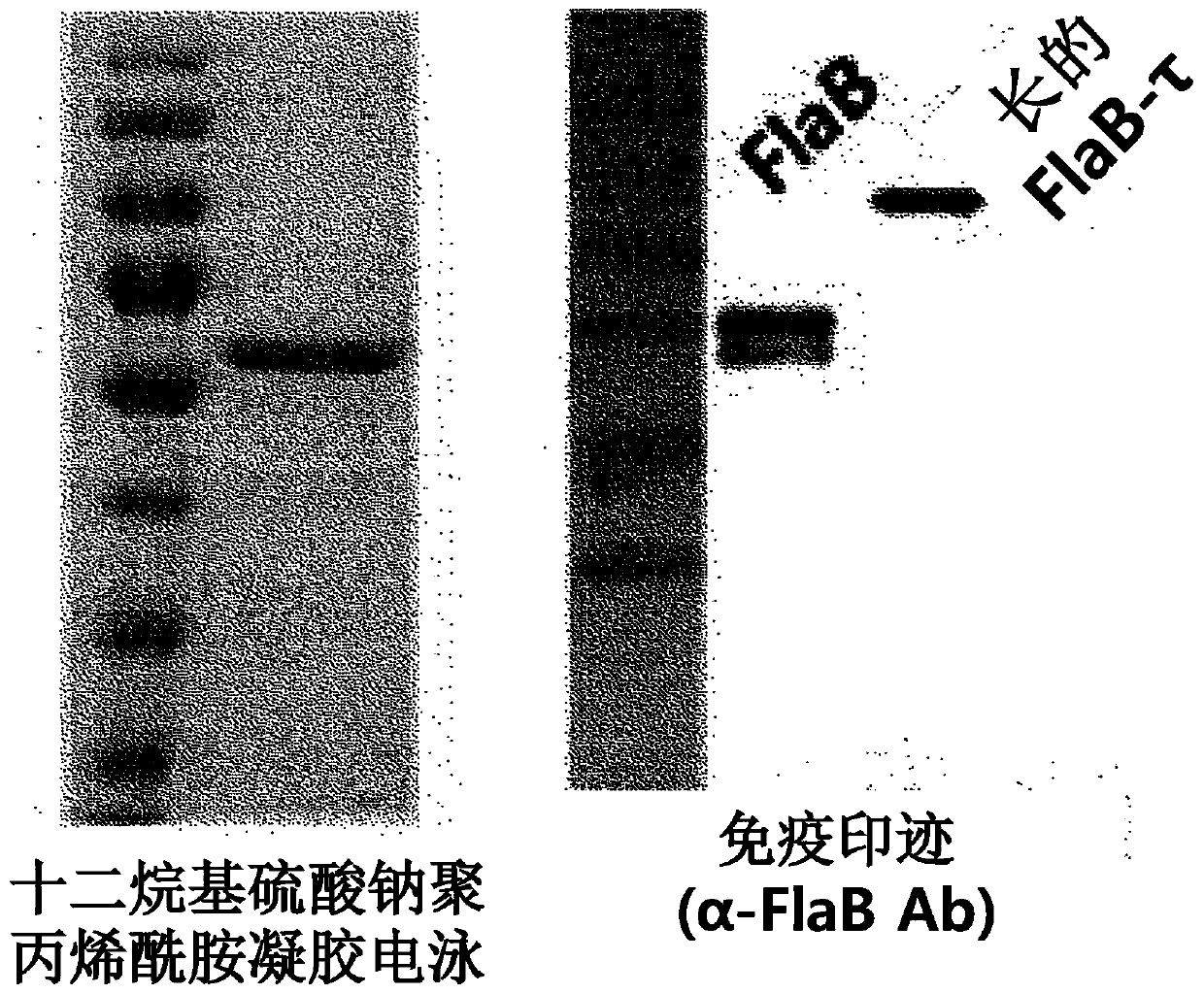
[0106] 1. Protein expression and purification
[0107] a. Expression and purification of FlaB recombinant protein derived from Vibrio vulnificus
[0108] The flaB recombinant protein was prepared by using the culture gene sequence (SEQ ID NO: 1) of the flagella constituent factor FlaB of Vibrio vulnificus CMCP6. A DNA fragment of 1.1 kbp comprising the FlaB gene for fusion N-terminus or for fusion C-te...
Embodiment 2
[0140] Embodiment 2: the preparation of norovirus immunization vaccine
[0141] a. Antigen sequence and codon optimization of Norovirus P domain
[0142] The DNA used to prepare the antigen for the preparation of the norovirus vaccine used pGEX-4T-1::VAxxx cloned with the norovirus P domain obtained from Professor Cho Kyung-woo of Chonnam University. Insert gene sequence such as SEQ ID NO:12.
[0143] b. Gene Cloning for Preparation of Recombinant Pd Antigen
[0144] In order to obtain the N-terminal of the Pd gene for the antigen or the product DNA fragment for the fusion C-terminal, by using the Pd-N and Pd-C primer pair described in SEQ ID NO:16 sequence 17, to contain The Pd plasmid of SEQ ID NO: 12 was used as a template to amplify a 1.1 kbp DNA fragment. That is, polymerase chain reaction using each primer pair was performed under the conditions of pre-denaturation at 95°C for 5 minutes, followed by 30 cycles of denaturation at 95°C for 30 seconds, annealing at 60°C f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com