COMBINATION THERAPY WITH AMIDINE SUBSTITUTED beta-LACTAM COMPOUNDS AND beta-LACTAMASE INHIBITORS FOR INFECTIONS WITH ANTIBIOTIC RESISTANT BACTERIAL STRAINS
A technology of lactamase and inhibitor, applied in the field of beta-lactam compounds, can solve problems such as multi-drug resistance and rare infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
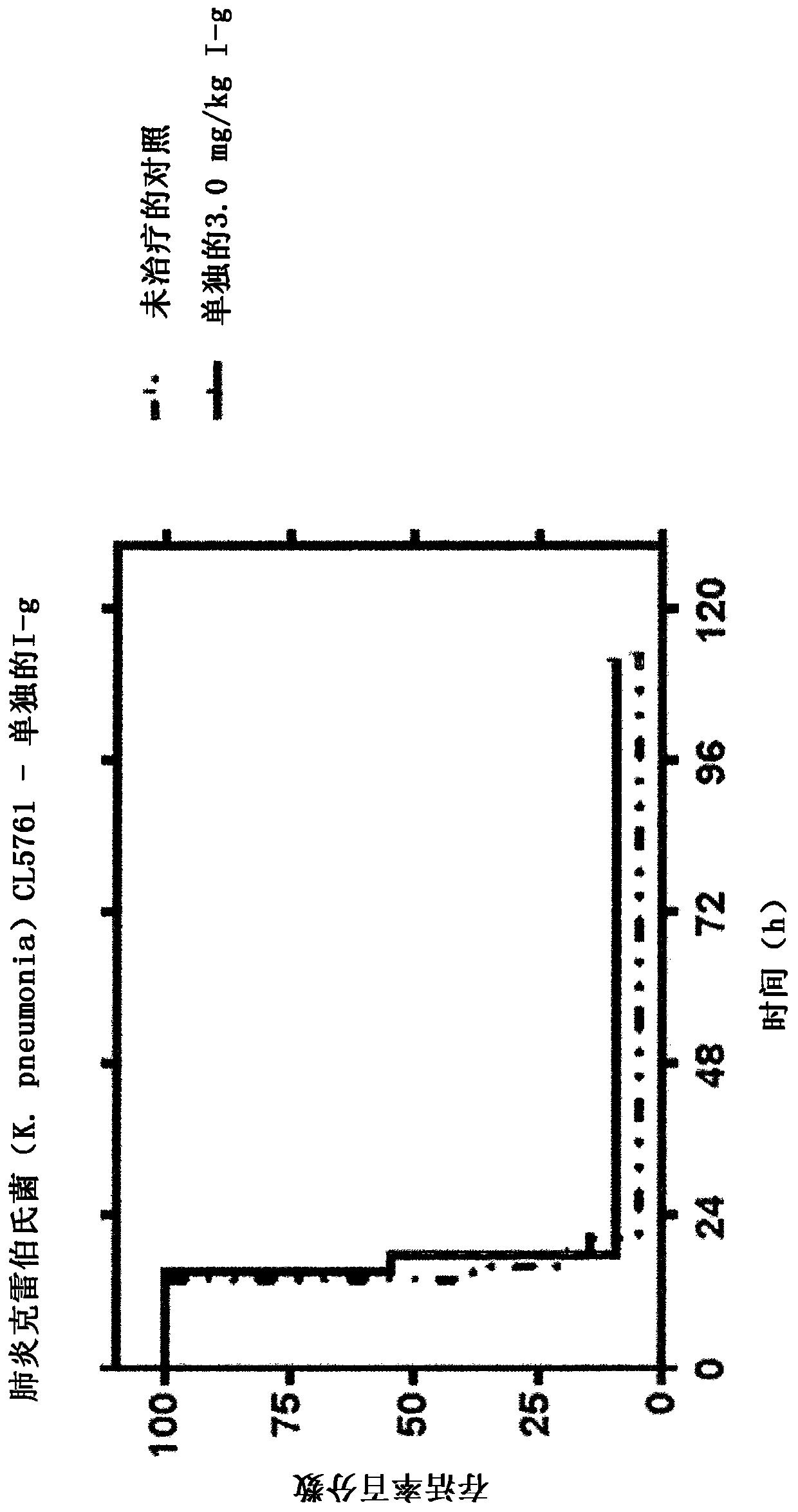
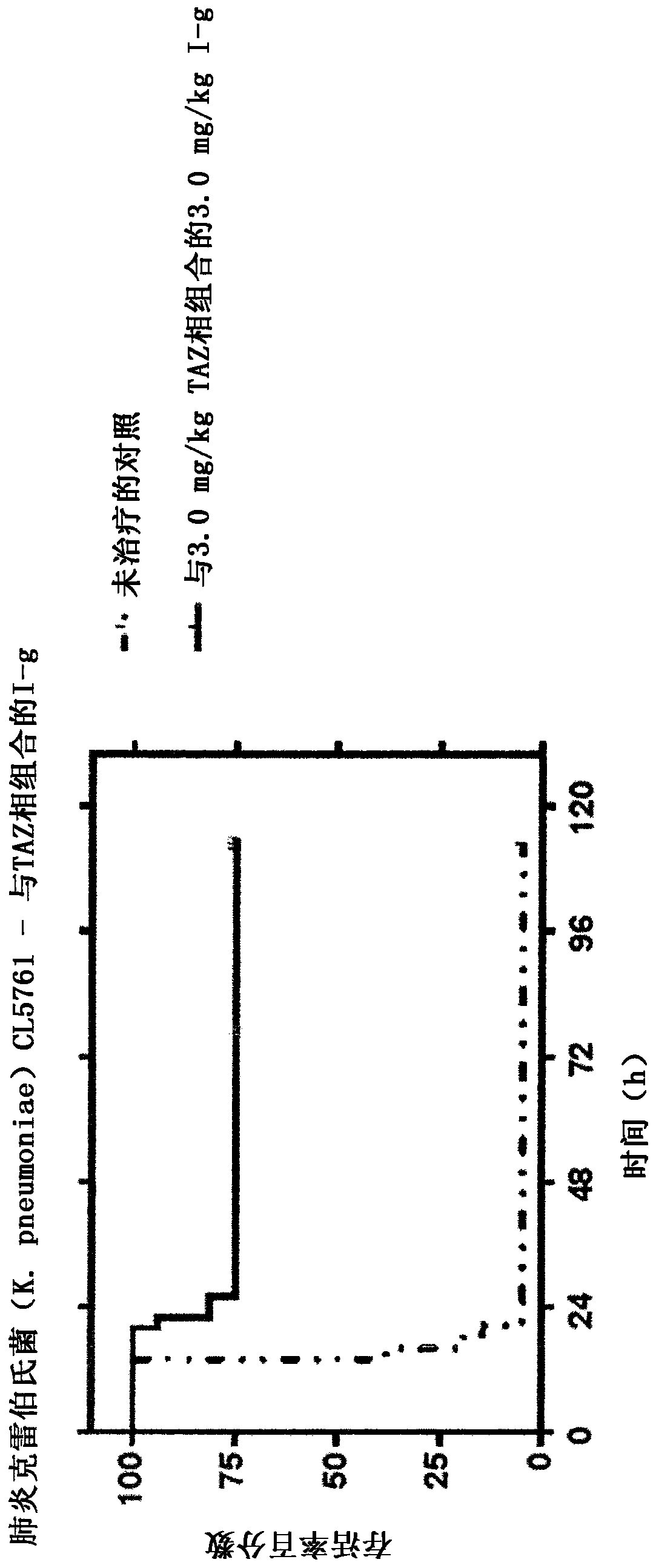
[0124] Compounds of formula (I-a, I-c, I-e and I-g) alone and in combination with clavulanic acid, tazobactam or sulbactam and alone and in combination with clavulanic acid, tazobactam or sulbactam The activity of the reference antibiotics against ESBL producing strains with β-lactamases belonging to different families (Table 1). Aztreonam was chosen as a reference for marketed monobactam antibiotics, ceftazidime as a reference for marketed cephalosporins, and piperacillin as a reference for marketed penicillin antibiotics (the latter can also be compared with BLI tazobactam obtained in combination).
[0125] Table 1 – Effects in ESBL producing strains
[0126]
[0127]
Embodiment 2
[0129] Analysis of compounds (I-a, I-c, I-e and I-g) in combination with clavulanic acid, tazobactam or sulbactam in strains producing ESBL and a different class of second β-lactamase. For clinical strains producing ESBLs and a second β-lactamase of a different class, it was surprisingly found that compounds of formula (I-a, I-c, I-e and I-g) + tazoba The combination of sulbactam or the compound of formula (I-a, I-c, I-e and I-g) + clavulanic acid or the combination of compound of formula (I-a, I-c, I-e and I-g) + sulbactam compared with the combination of the corresponding BLI The reference antibiotics all showed lower MICs.
[0130] Table 2 – Effects in ESBL+AmpC (Category C) co-producing strains
[0131]
[0132]
[0133] Table 3 – Effects in ESBL+KPC (class A carbapenemase) co-producing strains
[0134]
[0135]
[0136] Table 4 – Effects in ESBL+OXA-48 (D-class carbapenemase) co-producing strains
[0137]
[0138]
[0139] Table 5 – Effects in E...
Embodiment 3
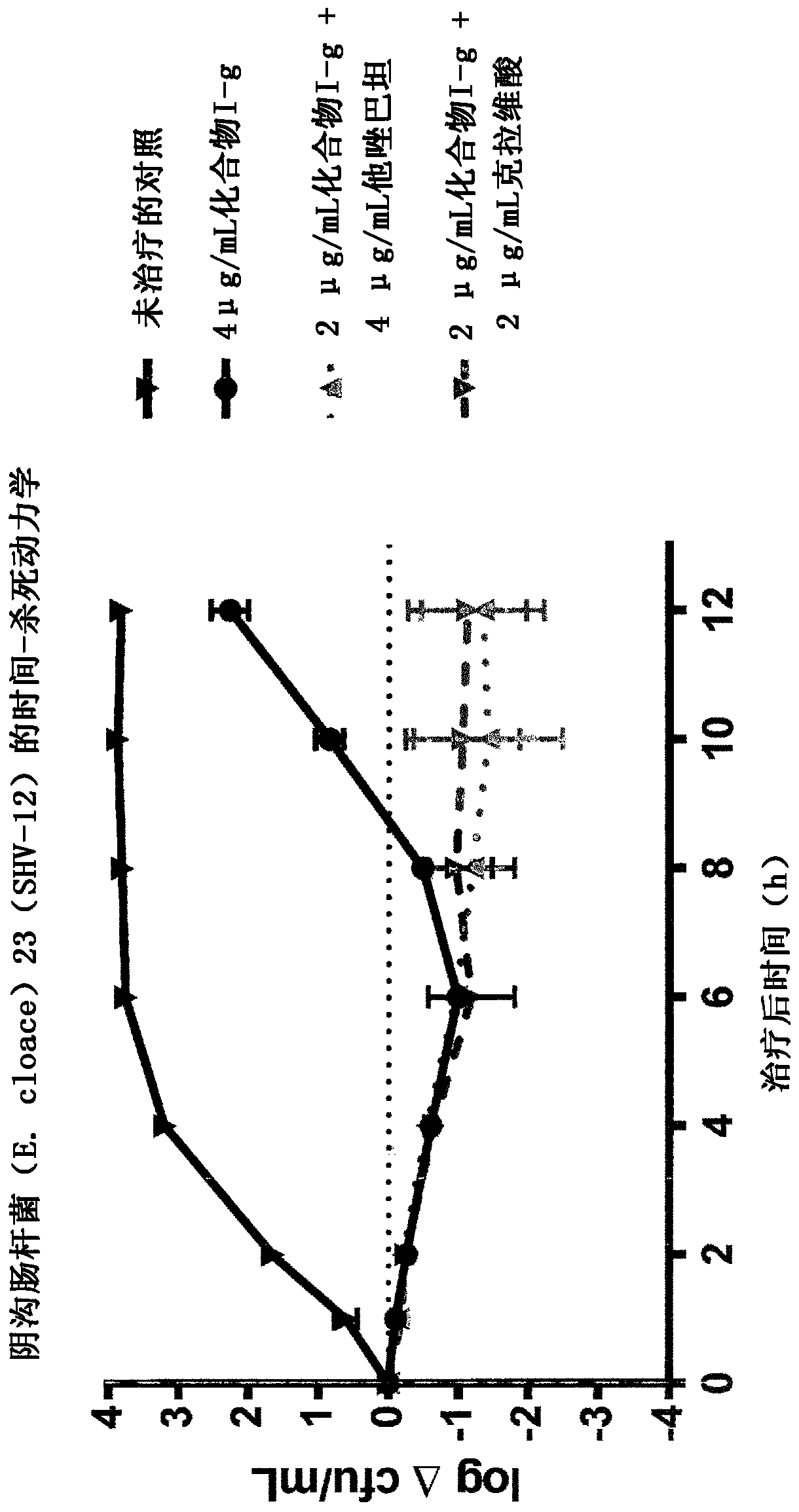
[0143] Time-kill kinetics of E. cloacae (E. cloacae) 23 (SHV-12) were produced using Compound I-g alone and in combination with 4 μg / mL tazobactam or 2 μg / mL clavulanic acid, The results are shown in figure 1 middle.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com