A kind of octafluoropentanol-based fluorine-containing surfactant and preparation method thereof
A surfactant, octafluoropentanol-based technology, applied in the field of octafluoropentanol-based fluorinated surfactants and its preparation, can solve the problem of difficult degradation of fluorinated surfactants and poor surface activity of octafluoropentanol-based , expensive and other issues, to achieve good application prospects, increase economic added value, and easy to degrade the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A kind of synthesis of octafluoropentyl alcohol-based fluorosurfactant: its reaction equation is as follows:
[0034]
[0035] 1) Take NaH (0.18mol, 7.2g) with a content of 60% and disperse it in a three-necked flask filled with 20mL tetrahydrofuran; dissolve 25.1mL (0.18mol) of octafluoropentanol in 30mL tetrahydrofuran. Slowly add it dropwise to a three-necked flask, and react at room temperature for 30 minutes after the dropwise addition, to obtain Compound A.
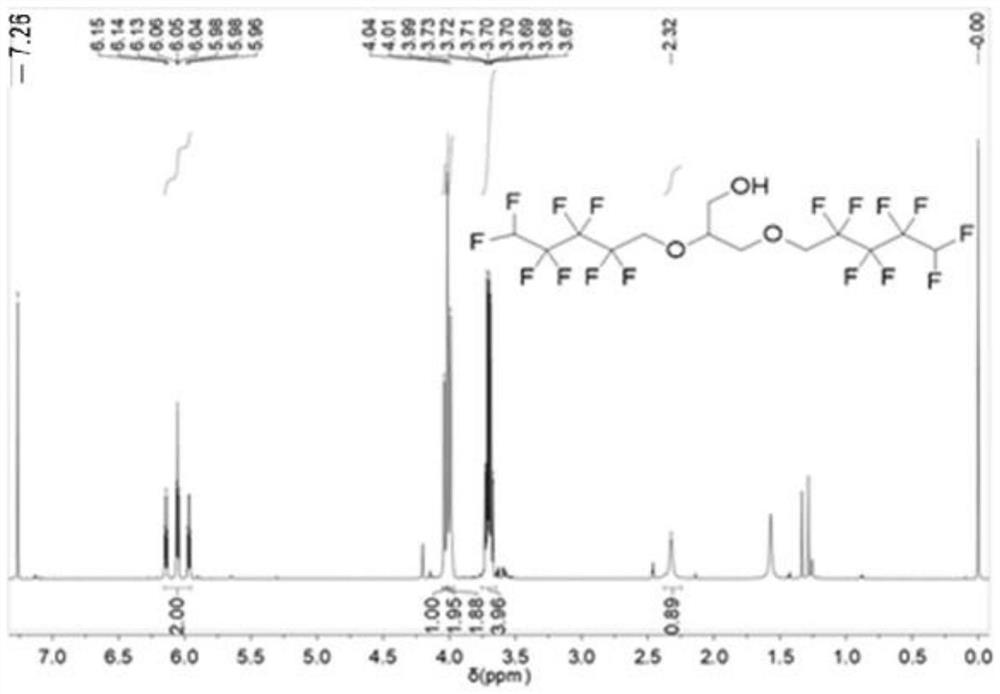
[0036] 2) Dissolve 2,3-dibromopropanol (6.11mL, 0.06mol) in 10mL tetrahydrofuran, and slowly add it dropwise to the compound A described in step 1). After the dropwise addition, raise the temperature to 50°C and react for 24h Compound B2 can be obtained. The obtained compound B2 is carried out to proton nuclear magnetic spectrum analysis, the result is as follows figure 1 shown. ( 1 H NMR (600MHz, CDCl 3 )δ6.05(tt, J=52.0,5.5Hz,2H),4.01(t,J=13.7Hz,5H),3.70(qd,J=9.7,5.1Hz,4H),2.33(d,J=5.2 Hz,1H).), th...
Embodiment 2
[0042] A kind of synthesis of octafluoropentyl alcohol-based fluorosurfactant: its reaction equation is as follows:
[0043]
[0044]1) Take NaH (0.18mol, 7.2g) with a content of 60% and disperse it in a three-necked flask filled with 20mL tetrahydrofuran; dissolve 25.1mL (0.18mol) of octafluoropentanol in 30mL tetrahydrofuran. Slowly add it dropwise to a three-necked flask, and react at room temperature for 30 minutes after the dropwise addition, to obtain Compound A.
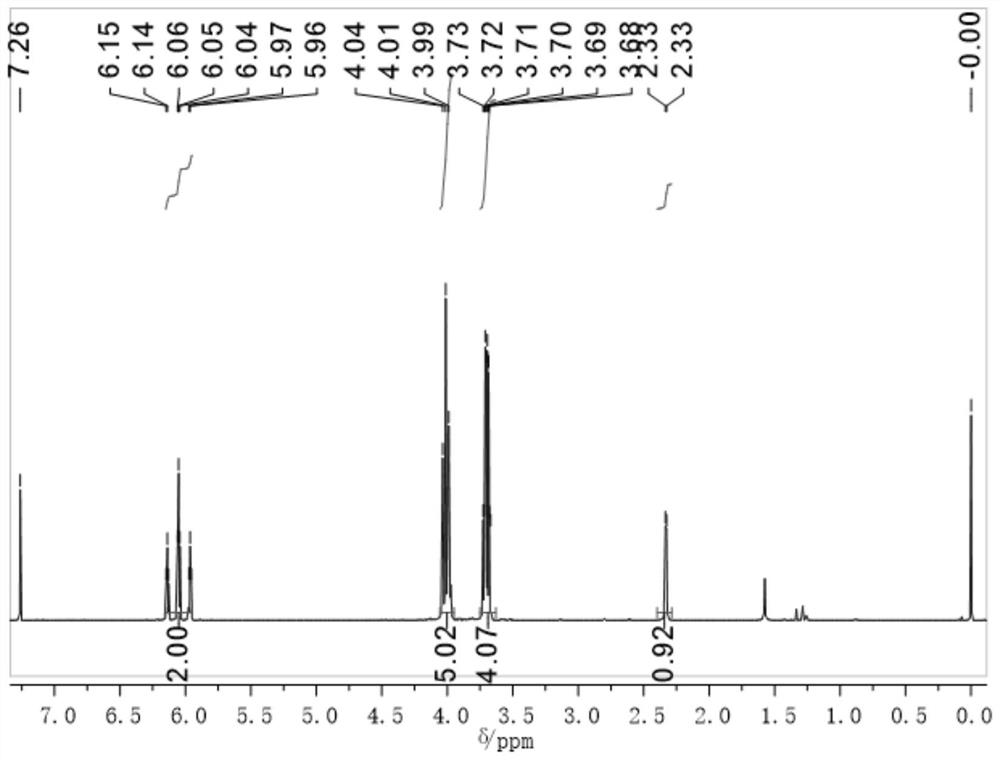
[0045] 2) Take 1,3-dibromo-2-propanol (6.11mL, 0.06mol) and dissolve it in 10mL tetrahydrofuran, slowly add it dropwise to the compound A described in step 1), and raise the temperature to 50°C after the dropwise addition is completed After 24 hours of reaction, compound B1 can be obtained. The obtained compound B1 is carried out to proton nuclear magnetic spectrum ( 1 H NMR (600MHz, CDCl 3 )δ6.15–5.95(m,2H),4.04(s,1H),4.01(s,2H),3.99(s,2H),3.70(qd,J=9.7,5.1Hz,4H),2.32(s ,1H).) and mass spectrometry ana...
Embodiment 3
[0051] A kind of synthesis of octafluoropentyl alcohol-based fluorosurfactant: its reaction equation is as follows:
[0052]
[0053] 1) Take NaH (0.18mol, 7.2g) with a content of 60% and disperse it in a three-necked flask filled with 20mL tetrahydrofuran; dissolve 25.1mL (0.18mol) of octafluoropentanol in 30mL tetrahydrofuran. Slowly add it dropwise to a three-necked flask, and react at room temperature for 30 minutes after the dropwise addition, to obtain Compound A.
[0054] 2) Dibromoneopentyl glycol (15.72g, 0.06mol) was dissolved in 20mL of tetrahydrofuran, and slowly added dropwise to the compound A described in step 1). After the dropwise addition, the temperature was raised to 50°C for 24 hours to obtain Compound B3.
[0055] 3) Dissolve 16.93g (0.03mol) of compound B3 in a three-necked flask filled with 50mL of tetrahydrofuran, slowly add 7.68g (0.066mol) of chlorosulfonic acid dropwise at room temperature, and react at room temperature for 24 hours to obtain fl...
PUM
| Property | Measurement | Unit |
|---|---|---|
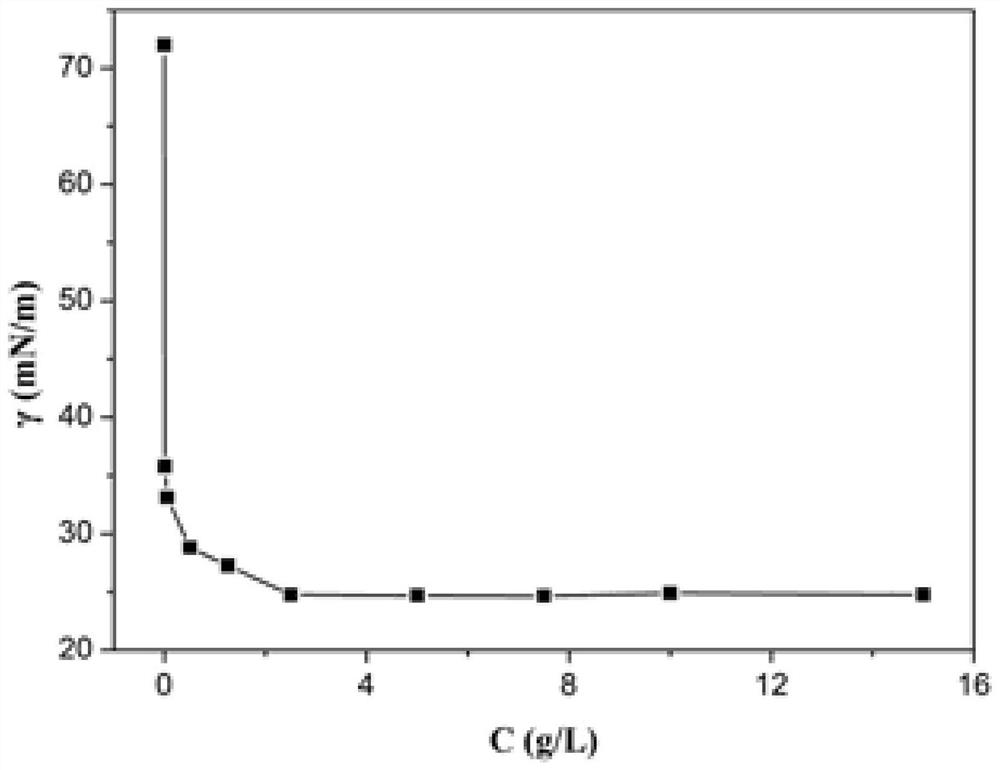
| critical surface tension | aaaaa | aaaaa |
| critical micelle concentration (mass) | aaaaa | aaaaa |
| critical surface tension | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com