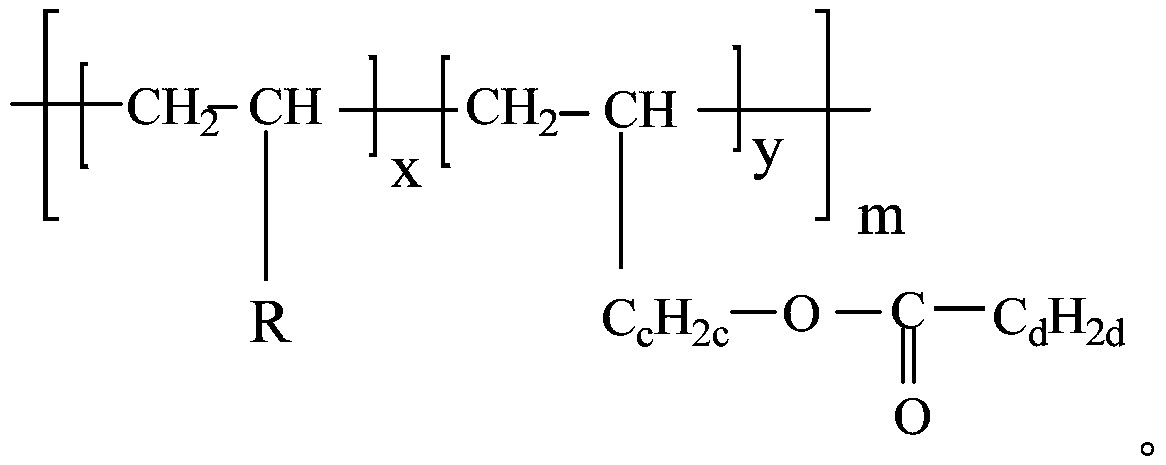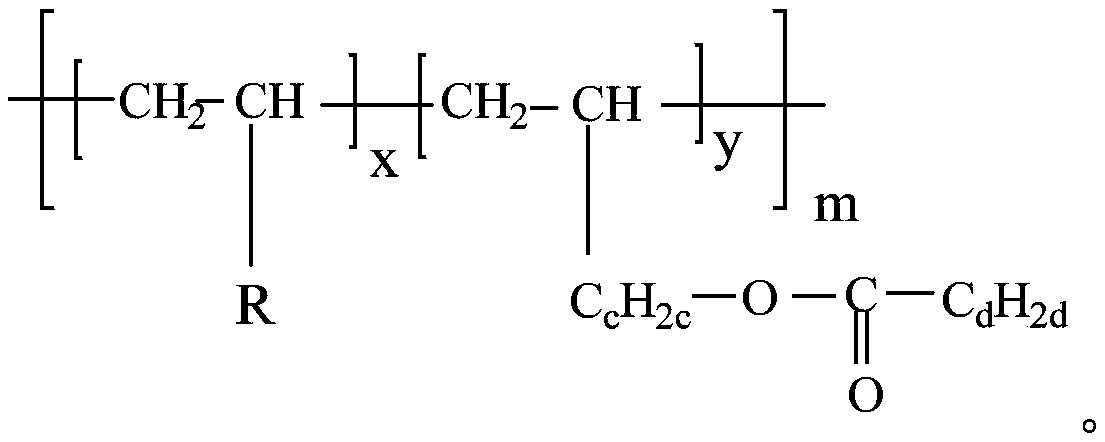Polyalphaolefin compound with polar ester group branched chain and preparation method thereof
A compound and alpha olefin technology, applied in the field of polyalpha olefin compounds and their preparation, can solve the problems of poor solubility of additives, and achieve the effects of improving anti-wear performance and enhancing polarity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] In a 250mL three-necked flask equipped with a stirrer, condenser and oil-water separator, add 0.62mol acrylic acid, 0.25mol 1,6-hexanediol, 0.745g (0.004mol) p-toluenesulfonic acid catalyst, 1.85g (0.017mol ) hydroquinone inhibitor and 59.4g (68.2mL, 0.64mol) toluene, react at 120°C for 5h, distill under reduced pressure to remove unreacted acrylic acid and residual water, stop heating, pour the reaction mixture in the flask into a beaker , neutralized and washed with 5% NaOH aqueous solution to remove hydroquinone, and then washed with water until neutral to obtain alkenyl ester.
[0041] Add the obtained alkenyl ester to the 1-decene polymerization reaction system, add the catalyst and heat at 30°C for 8h
[0042] Polymerization is carried out to obtain polyalphaolefin with polar ester branched chain, as shown below; in this step, the catalyst is AlCl 3 .
[0043]
[0044] Embodiment 1 Structural formula
Embodiment 2
[0046] In a 250mL three-necked flask with a stirrer, condenser and oil-water separator, add 0.40mol 9-decenoic acid, 0.50mol butanol, 0.42g (0.002mol) p-toluenesulfonic acid catalyst, 1.05g (0.009mol) Hydroquinone inhibitor, 42.06g (48.3mL, 0.46mol) toluene, react at 140°C for 12h, distill off unreacted butanol and residual water under reduced pressure, stop heating, pour the reaction mixture in the flask into a beaker , neutralized and washed with 5% NaOH aqueous solution to remove hydroquinone, and then washed with water until neutral to obtain alkenyl ester.
[0047] Add the obtained alkenyl ester to the 1-dodecene polymerization reaction system, add a catalyst and heat at 70°C for 4 hours to carry out the polymerization reaction to obtain a polyalphaolefin with polar ester branched chains, as shown below; in this step , the catalyst is Ziegler-Natta.
[0048]
[0049] Embodiment 2 Structural formula
Embodiment 3
[0051] In a 250mL three-necked flask equipped with a stirrer, condenser and oil-water separator, add 0.60mol propylene alcohol, 0.50mol lauric acid, 1.35g (0.008mol) p-toluenesulfonic acid catalyst, 0.68g (0.006mol) terephthalic acid Phenol inhibitor and 40.5g (46.6mL, 0.44mol) toluene, react at 160°C for 4h, distill off unreacted allyl alcohol and residual water under reduced pressure, stop heating, pour the reaction mixture in the flask into a beaker, and use 5 % NaOH aqueous solution to neutralize and wash to remove hydroquinone, and then wash with water until neutral to obtain alkenyl ester.
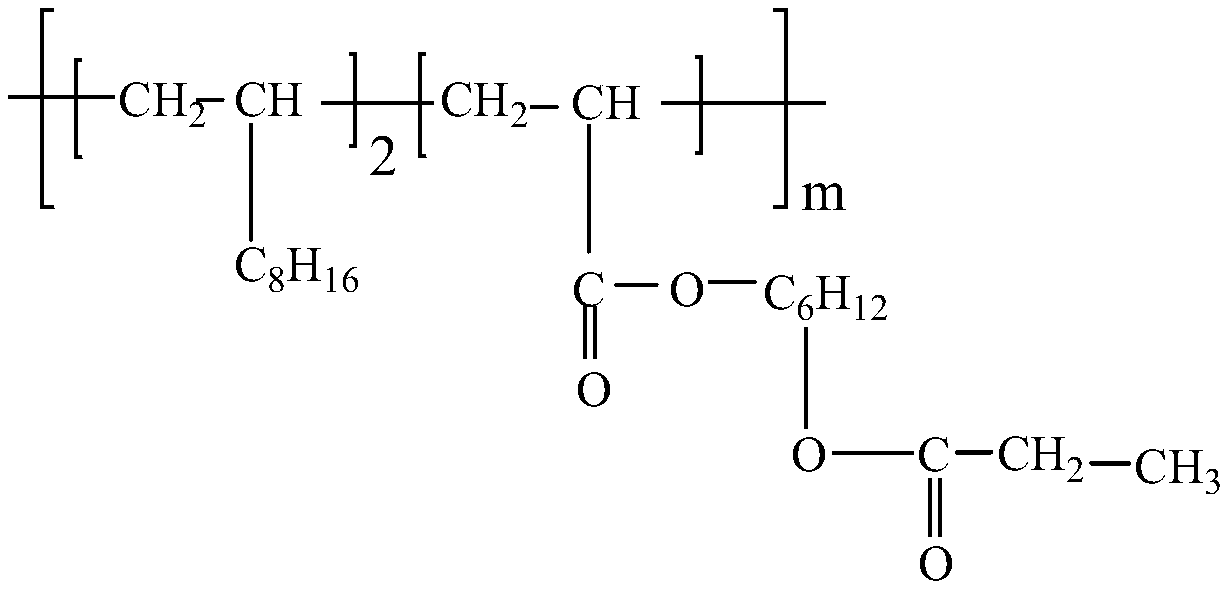
[0052] Add the obtained alkenyl ester to the 1-decene polymerization reaction system, add a catalyst and heat at 100°C for 4 hours to carry out the polymerization reaction, and obtain a polyalphaolefin with polar ester branched chains, as shown below; in this step, the catalyst for metallocenes.
[0053]
[0054] Embodiment 3 Structural formula
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com