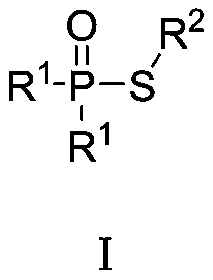
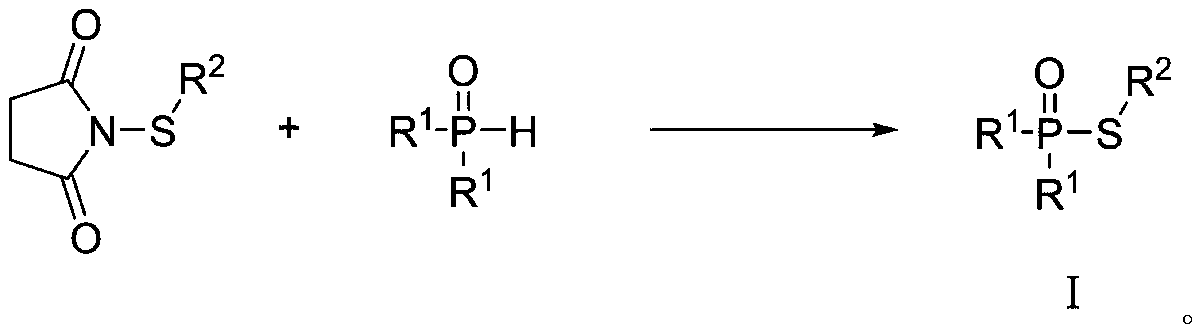
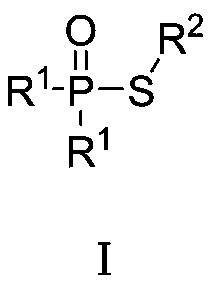
Green synthetic method of thiohypophosphate
A technology of thiophosphinate and synthesis method, which is applied in the fields of chemical instruments and methods, compounds of group 5/15 elements of the periodic table, organic chemistry, etc., can solve problems such as limiting wide application, and achieve the effect of great application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0010] Embodiment 1: Preparation of diphenylthiophosphinic acid-S-phenyl ester (compound number 1)
[0011]
[0012] Add N-phenylthiophthalimide (207mg, 1.0mmol) and diphenylphosphine oxide (202mg, 1.0mmol) into 5mL of water, stir and react at 40°C for 3h, filter, wash with water, and air-dry. 245 mg of diphenylthiophosphinic acid-S-phenyl ester was obtained (yield: 79%).
[0013] 1 H NMR (400MHz, CDCl 3 ):δ7.79-7.72(m,4H),7.55-7.50(m,6H),7.38-7.34(m,2H),7.28-7.22(m,3H);LC / MS(M+1) + = 311.1.
Embodiment 2
[0014] Embodiment 2: Preparation of two (4-methylphenyl) thiophosphinic acid-S-phenyl ester (compound number 2)
[0015]
[0016] Add N-phenylthiophthalimide (207mg, 1.0mmol) and bis(4-methylphenyl)phosphine oxide (230mg, 1.0mmol) into 5mL water, stir at 40°C for 2h, filter, Washed with water and air-dried to obtain 284 mg of bis(4-methylphenyl)thiophosphinic acid-S-phenyl (yield: 84%).
[0017] 1 H NMR (400MHz, CDCl 3 ):δ7.76-7.72(m,4H),7.54-7.49(m,6H),7.27-7.22(m,3H),2.39(s,6H); LC / MS(M+1) + = 339.1.
Embodiment 3
[0018] Example 3: Preparation of two (3,5-dimethylphenyl) thiophosphinic acid-S-phenyl ester (compound number 3)
[0019]
[0020] Add N-phenylthiophthalimide (207mg, 1.0mmol) and bis(3,5-dimethylphenyl)phosphine oxide (258mg, 1.0mmol) into 5mL of water, and stir at 40°C for 2h , filtered, washed with water, and air-dried to obtain 315 mg of bis(3,5-dimethylphenyl)thiophosphinic acid-S-phenyl ester (yield: 86%).
[0021] 1 H NMR (400MHz, CDCl 3 ):δ7.61-7.55(m,6H),7.39-7.36(m,2H),7.27-7.23(m,3H),2.39(s,6H),2.35(s,6H); LC / MS(M +1) + = 367.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com