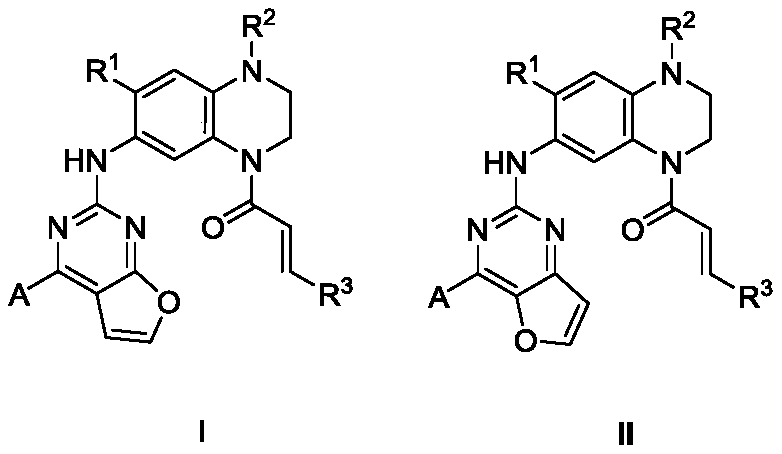
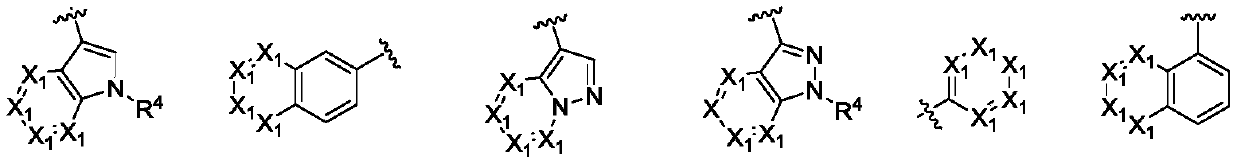
Furo pyrimidin di-aromatic nucleus derivative epidermal growth factor inhibitor and preparation method and applications thereof
A technology of epidermal growth factor and pyrimidine, which is applied in the field of bisaromatic ring derivatives of furopyrimidine, can solve the problems of cancer recurrence in patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: 2-{1'-N,N-dimethylaminoethyl-4'-acrylamido-7'-methoxy-1',2',3',4'-tetrahydro- 6'-quinoxaline}-4-(1"-methyl-1H-3"-indole)furo[2,3-D]pyrimidine (compound 401)
[0034]
[0035] Preparation of 2-chloro-4-(1'-methyl-1H-3'-indole)furo[2,3-D]pyrimidine:
[0036]
[0037] Dissolve 2,4-dichlorofuro[2,3-D]pyrimidine (0.75g, 4mmol) in ethylene glycol dimethyl ether (20mL), stir under ice bath, add ferric chloride (0.77 g, 4.59mmol), followed by stirring the reaction at room temperature for 15 minutes. Then N-methylindole (0.68 g, 5.2 mmol) was added dropwise, followed by heating to 60°C and stirring for 24 hours. Stop the reaction, lower to 0°C, add 3.5mL of methanol and 9mL of water, then stir the reaction at room temperature for 3 hours. A large amount of solid precipitated, filtered, washed the filter cake with methanol, and dried to obtain 0.82 g of yellow solid, yield: 72%. LC / MS (ESI): m / z 284 (M+H) + .
[0038] Preparation of tert-butyl 4-fluoro-2-me...
Embodiment 2
[0062] Example 2: 2-{1'-N,N-Dimethylaminoethyl-4'-acrylamido-7'-methoxy-1',2',3',4'-tetrahydro-6 '-quinoxaline}-4-(1"-methyl-4-aza-3"-indole)furo[3,2-D]pyrimidine (Compound 402)
[0063]
[0064] Preparation of 2-chloro-4-(1'-methyl-1H-3'-indole)furo[3,2-D]pyrimidine:
[0065]
[0066] Dissolve 2,4-dichlorofuro[3,2-D]pyrimidine (0.75g, 4mmol) in ethylene glycol dimethyl ether (20mL), stir under ice bath, add ferric chloride (0.77 g, 4.59 mmol), followed by stirring the reaction at room temperature for 15 minutes. Then N-methylindole (0.68 g, 5.2 mmol) was added dropwise, followed by heating to 60°C and stirring for 24 hours. Stop the reaction, lower to 0°C, add 3.5mL of methanol and 9mL of water, then stir the reaction at room temperature for 3 hours. A large amount of solid precipitated, filtered, washed the filter cake with methanol, and dried to obtain a yellow solid, 0.86 g, yield: 76%. LC / MS (ESI): m / z 284 (M+H) + .
[0067] 2-[1'-N,N-Dimethylaminoethyl-7'-metho...
Embodiment 3
[0073] Example 3: 2-{1'-N,N-dimethylaminoethyl-4'-butenamido-7'-methoxy-1',2',3',4'-tetrahydro- 6'-quinoxaline}-4-(3"-pyrazol[1,5-a]pyridine)furo[2,3-D]pyrimidine (Compound 403)
[0074]
[0075] 2-{1'-N,N-Dimethylaminoethyl-4'-acrylamido-7'-methoxy-1',2',3',4'-tetrahydro-6'-quinoxa Preparation of morpholine}-4-(3"-pyrazol[1,5-a]pyridine)furo[2,3-D]pyrimidine (compound 403):
[0076]
[0077] 2-[1'-N,N-Dimethylaminoethyl-7'-methoxy-1',2',3',4'-tetrahydro-6'-quinoxaline]-4-( 3"-pyrazolo[1,5-a]pyridine)furanopyrimidine (378mg, 0.76mmol) and DIPEA (0.146mL, 0.84mmol) were dissolved in dichloromethane (10mL), stirred at 0°C, and then dropped Add crotonoyl chloride (79mg, 0.76mmol) and dissolve in DCM (2mL) solution, then stir and react for 2 hours. The reaction is complete as detected by TLC. Stop the reaction, add DCM (50mL), then use 100mL saturated NaHCO 3 Washed with water, the aqueous layer was extracted with DCM (2*50mL), anhydrous MgSO 4 After drying and concentra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com