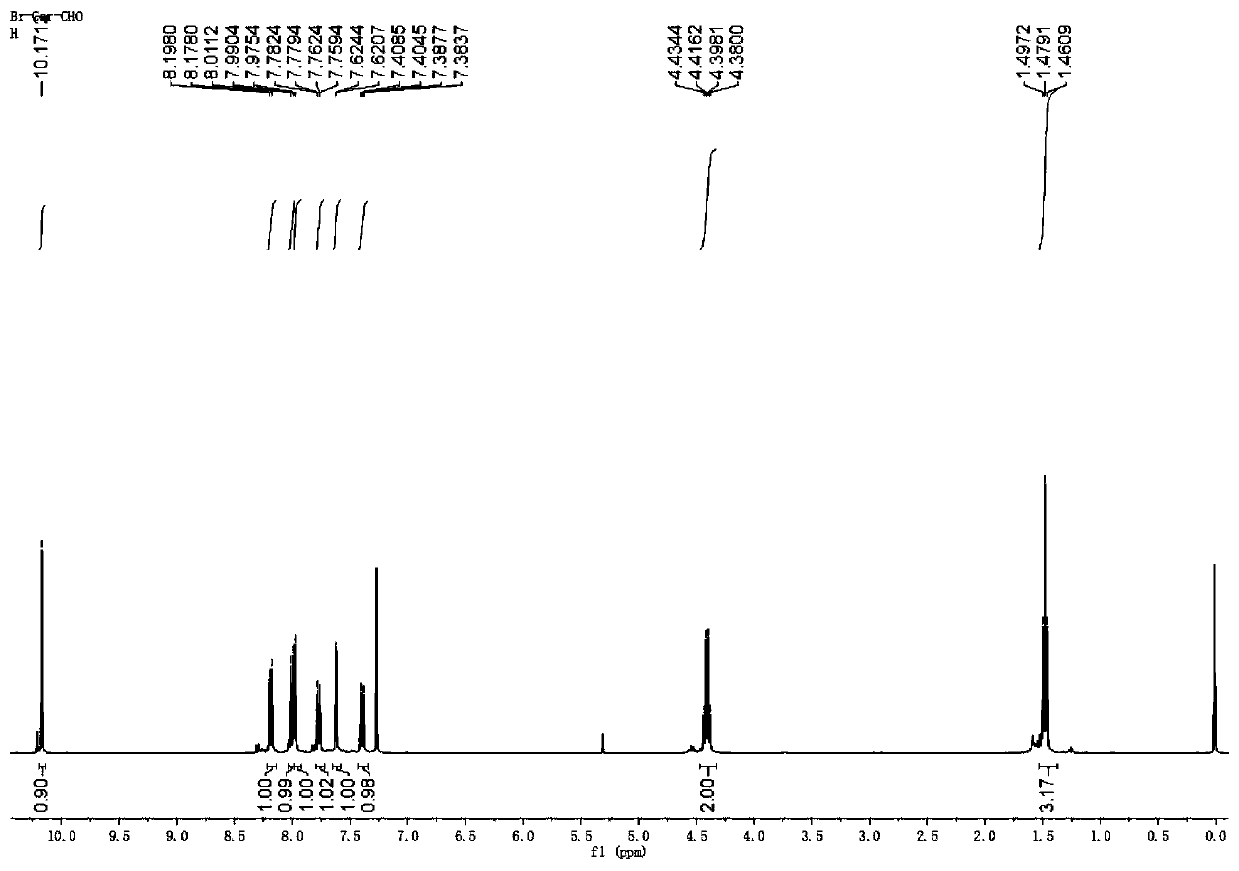
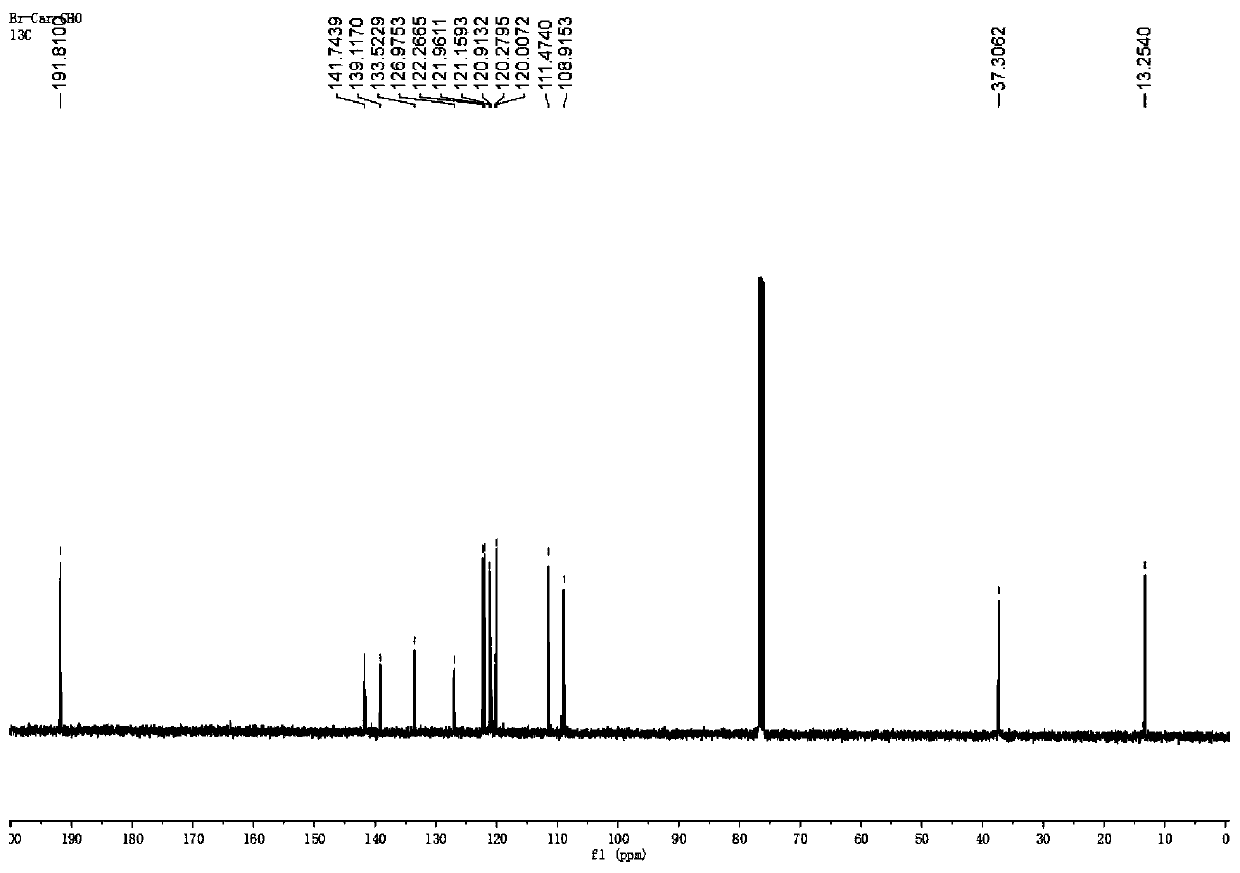
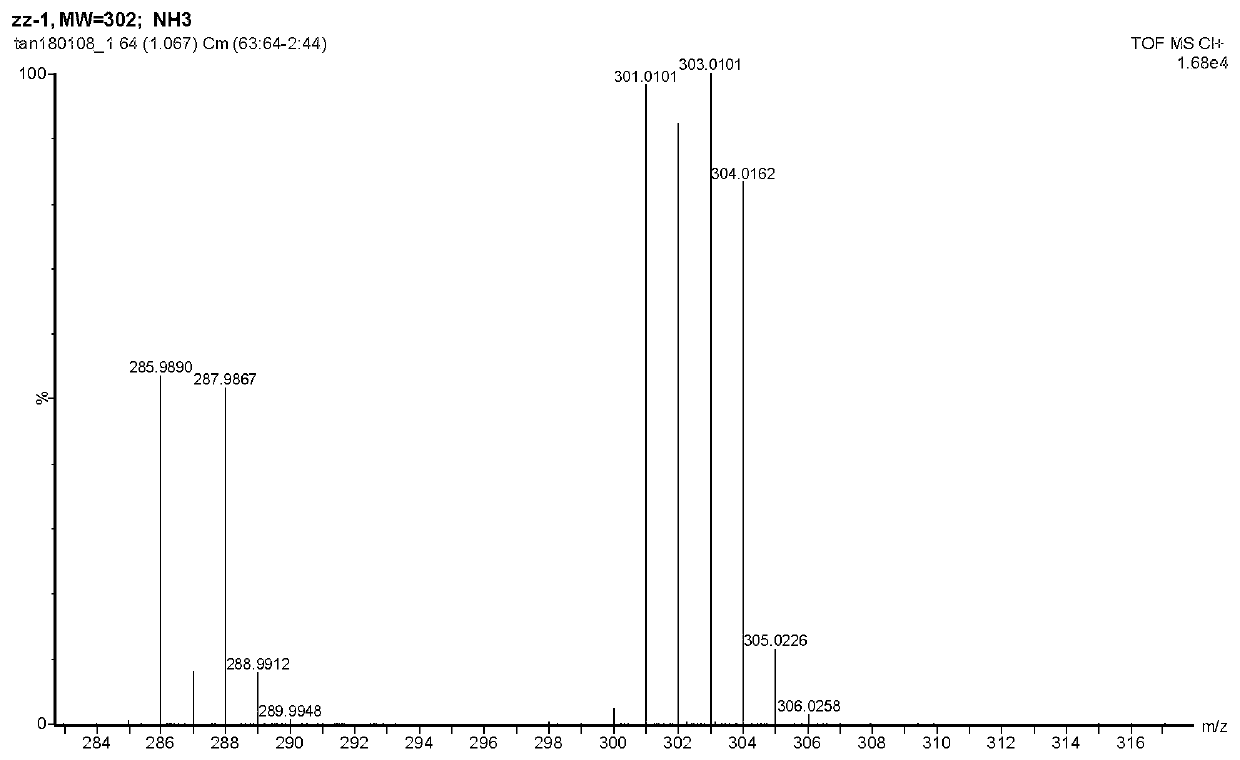
Preparation and application of near-infrared compound with strong two-photon absorption
A two-photon absorption and compound technology, which is applied to medical preparations containing active ingredients, organic chemistry, preparations for in vivo experiments, etc., can solve poor photostability, small Stokes shift, two-photon organelle fluorescence imaging And treat problems such as a handful, to achieve the effect of high contrast and high photostability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0065] In order to have a clearer understanding of the technical features, purposes and effects of the present invention, the specific implementation manners of the present invention will now be described in detail with reference to the accompanying drawings. Apparently, the described embodiments are only some, not all, embodiments of the present invention. Based on the implementations described in this specification, all other implementations obtained by persons of ordinary skill in the art without making creative efforts fall within the protection scope of the present invention.
[0066] The present invention provides a variety of near-infrared aggregation-induced fluorescent materials with strong two-photon absorption, and the fluorescent materials have a structure shown in the following chemical formula (I):
[0067]
[0068] Wherein, R is an electron acceptor, and the electron acceptor is selected from one of the following structural formulas:
[0069]
[0070] Fur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com