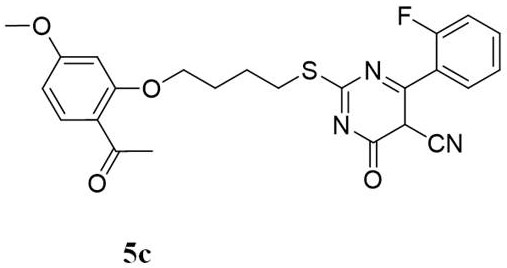
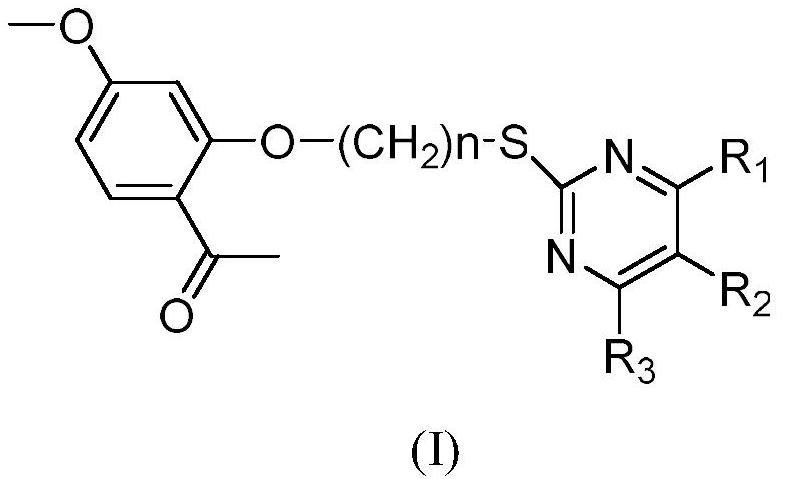
Paeonol dihydropyrimidinone derivatives and preparation method and application thereof
A technology of paeonol dihydropyrimidinone and derivatives is applied in the field of paeonol dihydropyrimidinone derivatives and preparation thereof, and can solve the problems that no public reports of dihydropyrimidinone derivatives have been reported, and achieves The effect of high purity, improved antitumor activity, and short preparation cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
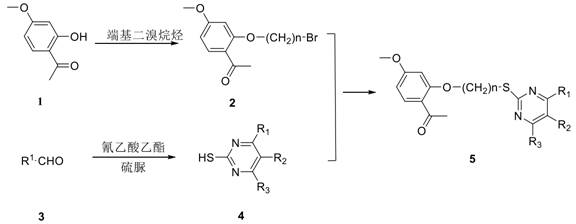
[0045] Example 1: Preparation of 2-(4-bromobutoxy)-4-methoxyacetophenone (Compound 2)
[0046] 15.0 mmol of paeonol 1 and 15.0 mmol of anhydrous potassium carbonate were added to a 250 ml round-bottomed flask, dissolved in 150 mmol of acetone, and 48.0 mmol of 1,4-dibromobutane was added under stirring at room temperature, and the reaction was stirred at room temperature. To 16 hours, TLC was performed to track the progress of the reaction (V 石油醚 :V 乙酸乙酯 =4:1) After the reaction is completed, dry and filter the filtrate, evaporate the filtrate to dryness under reduced pressure, and then pass through silica gel column chromatography (V 石油醚 :V 乙酸乙酯 =10:1) Purified to obtain 2.60 g of bromopaeonol as a white solid 2, Rf=0.28, yield 58.0%.
[0047] m.p.51.4~53.0℃; IR(KBr,cm -1 ):2956(-CH 3 ), 1651 (C=O), 1595, 1500, 1406, 1359, 1328, 1257, 1124, 1024, 837, 756, 646, 584, 522;
[0048] Therefore, the above compound 2 is 2-(4-bromobutoxy)-4-methoxyacetophenone, and its structu...
Embodiment 2
[0050] Example 2: Preparation of 6-Substituted Phenyl-5-cyano-2-thiouracil (Compound 4)
[0051] Add 0.01 mol of aromatic aldehyde, 0.01 mol of ethyl cyanoacetate, 0.01 mol of thiourea and 0.01 mol of potassium carbonate to 0.1 mol of absolute ethanol solution and reflux for 5-8 h, during which the reaction progress is detected by TLC; Precipitate in the form of precipitation, filter under reduced pressure, dissolve the precipitate in warm water, acidify it with acetic acid and re-precipitate, filter under reduced pressure, dry it, recrystallize from ethanol, and purify to obtain 6-substituted phenyl-5-cyano- 2-thiouracil;
[0052] Its structural formula is shown in the following formula, wherein, R 1 is aryl, H or CH 3 ;R 2 is CN or H; R 3 as OH or CH 3 ;
[0053]
[0054] The 4a is 6-(3-fluorophenyl)-5-cyano-2-thiouracil;
[0055] 4b is 6-phenyl-5-cyano-2-thiouracil;
[0056] 4c is 6-(2-fluorophenyl)-5-cyano-2-thiouracil;
[0057] 4d is 6-(3-hydroxyphenyl)-5-cyan...
Embodiment 3
[0065] Example 3: Preparation of 6-(3-Fluorophenyl)-5-cyano-4-hydroxy-2-paeonol mercaptopyrimidine (5a)
[0066] Get the 2-(4-bromobutoxy)-4-methoxyacetophenone 2 of 1mmol prepared in Example 1, be dissolved in the round-bottomed flask that 10mmol absolute ethanol is housed, add 1mmol of 6-( 3-Fluorophenyl)-5-cyano-2-thiouracil 4a, 1 mmol of anhydrous potassium carbonate was added, the temperature was raised to 78°C in a water bath, and the reaction was stirred at reflux for about 7 to 8 hours, during which TLC was performed to track the progress of the reaction (V 氯仿 :V 甲醇 =2:1) After the reaction is completed, dry and filter, the filtrate is evaporated to dryness under reduced pressure and subjected to silica gel column chromatography (V 石油醚 :V 乙酸乙酯 =1:1) purification to obtain white solid 5a, yield 32.1%;
[0067] m.p.81.7~83.6℃; IR (KBr,cm -1 ): 2 947 (CH 3 ), 2 214(-CN), 1664(C=O), 1600, 1502, 1485, 1396, 1357, 1303, 1263, 1199, 1153, 1028, 999, 964, 796, 729, 646,...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap