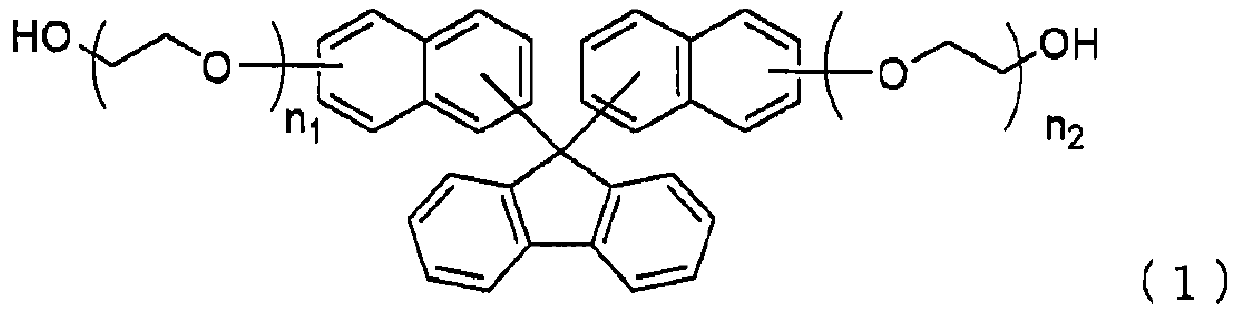
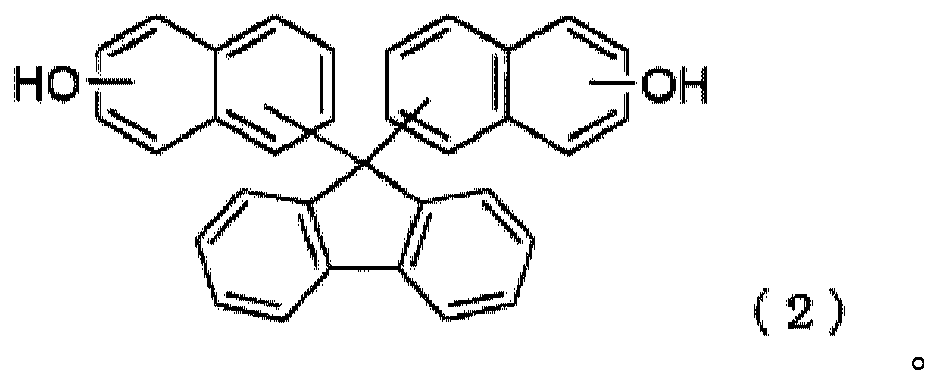
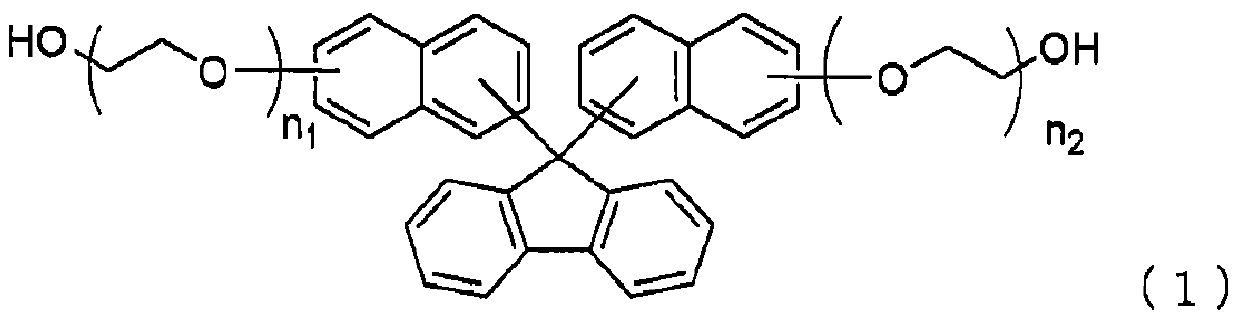
Method for producing alcohols having fluorene skeleton
A manufacturing method and technology of alcohols, applied in ether preparation, organic chemistry, ester reaction to prepare ethers, etc., can solve problems such as unknown manufacturing methods of alcohols, and achieve the effect of reducing the quantity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0135] Add 30.0 g (0.17 mol) of 9-fluorenone, 57.6 g (0.40 mol) of 2-naphthol, and 1.79 g (0.008 mol), toluene 45.0g, γ-butyrolactone 14.8g and phosphotungstic acid 0.8g, decompressed to 49.3kPa, then raised the temperature to 100°C, stirred at the same temperature for 7 hours, and confirmed 9-fluorenone by HPLC The residual rate is less than 0.2%.
[0136] Next, 0.9 g of a 24% aqueous sodium hydroxide solution (hereinafter also referred to as caustic water) was added to the obtained reaction liquid to neutralize phosphotungstic acid, and then the temperature was raised to 120° C. to distill water.
[0137] Then, 1.2 g of potassium carbonate, 36.6 g of ethylene carbonate, and 1.5 g of toluene were added to the reaction solution, the temperature was raised to 110° C., and then stirred at the same temperature for 13 hours. It was confirmed by HPLC that the formula (2-1) represented The disappearance of bis-naphthol compounds.
[0138] After completion of the reaction, 7.5 g of...
Embodiment 2
[0144] Add 30.0 g (0.17 mol) of 9-fluorenone, 57.6 g (0.40 mol) of 2-naphthol, and 1.79 g (0.008 mol), toluene 30.0g, ethyl acetate 30.0g and phosphotungstic acid 0.8g, decompressed to 56.7kPa, then raised the temperature to 100°C, stirred at the same temperature for 4 hours, and confirmed the residue of 9-fluorenone by HPLC rate of 0.2% or less.
[0145] Next, 0.9 g of 24% caustic water was added to the obtained reaction liquid to neutralize phosphotungstic acid, and then the temperature was raised to 120° C. to distill water.
[0146] Then, 1.2 g of potassium carbonate, 36.6 g of ethylene carbonate, and 1.5 g of toluene were added to the reaction liquid, the temperature was raised to 110° C., and then stirred at the same temperature for 16 hours, and the formula (2-1) represented by the above-mentioned formula (2-1) was confirmed by HPLC. Disappearance of bis-naphthol compounds.
[0147] After completion of the reaction, 7.5 g of water and 26.3 g of 24% caustic water were ...
Embodiment 3
[0151] Except having changed the ethyl acetate in Example 2 into butyl acetate, it carried out similarly, and obtained the alcohol compound represented by said formula (1-1). The yield, yield and purity of the obtained alcohol compound represented by the above formula (1-1) are shown below.
[0152] Weight of obtained crystals: 80.9 g (yield: 90.2%)
[0153] HPLC purity: 94.6%
PUM
| Property | Measurement | Unit |
|---|---|---|
| Residual rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com