Carbazole sulfonamide derivative prodrug or medicinal salts thereof and preparation method and applications thereof
A technology of carbazole sulfonamide and medicinal salt, which is applied in the field of biopharmaceuticals and can solve problems such as complex chemical structure, neurotoxicity, and difficult chemical total synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
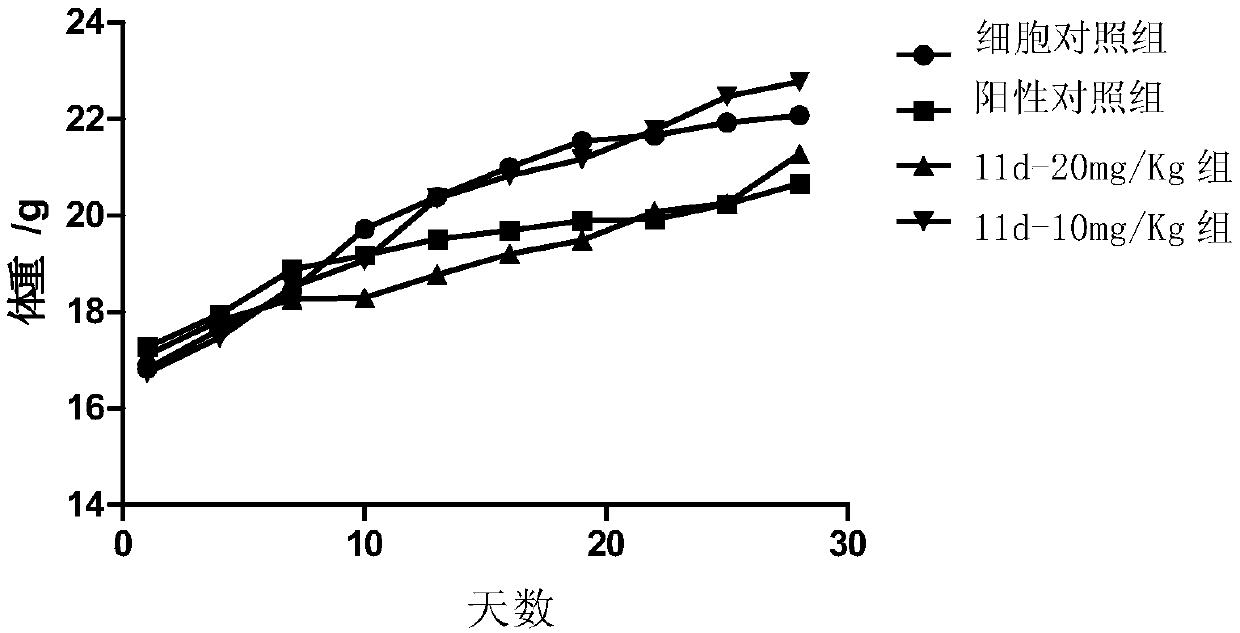
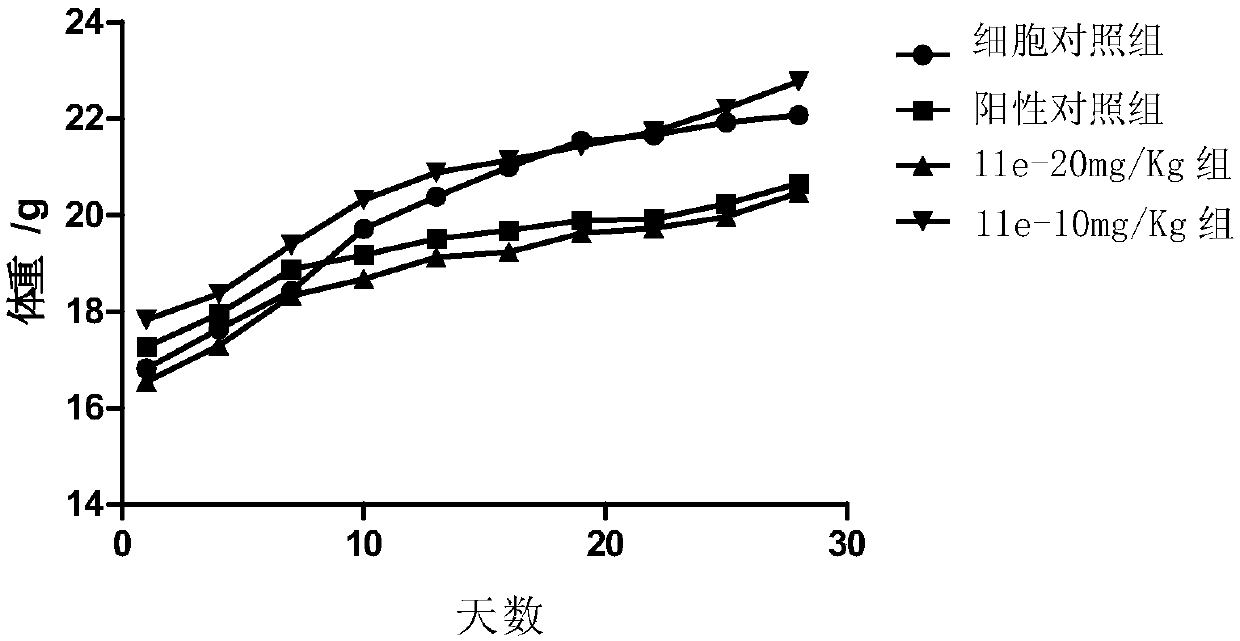
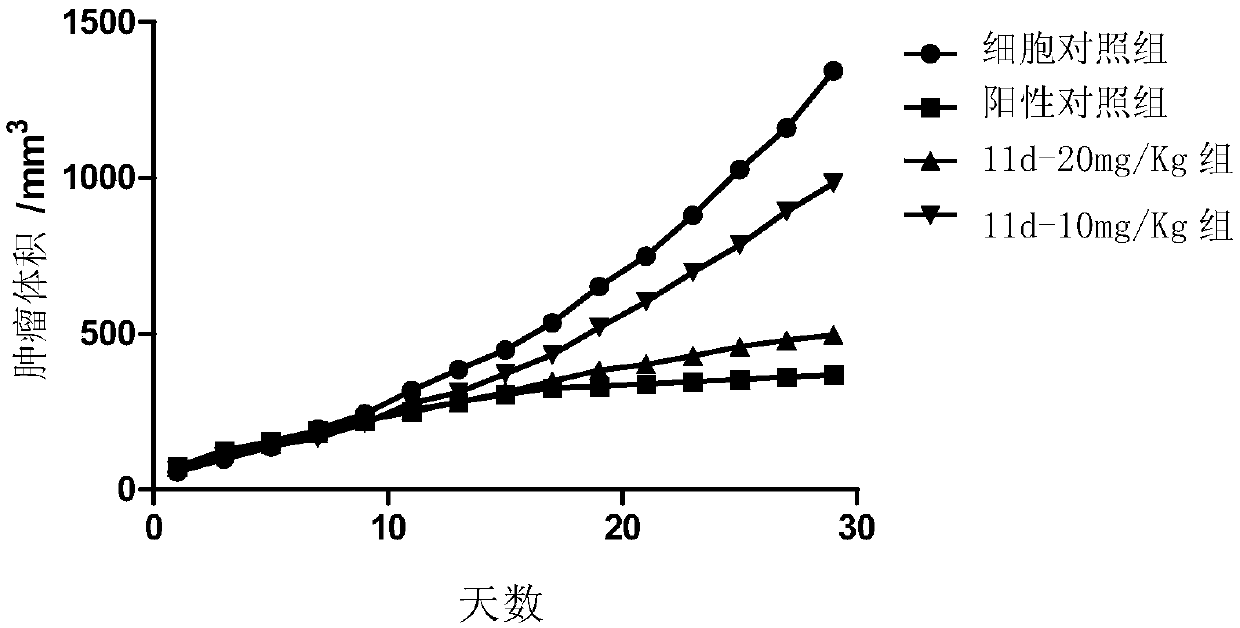
Image
Examples
Embodiment 1
[0080] (S)-N-(2,6-dimethoxypyridin-3-yl)-2-(2-amino-3-(4-hydroxy)phenyl-propionylamino)-N-methyl-6 -Synthesis of carbazole sulfonamide hydrochloride (11a)
[0081] Step (1), synthesis of reaction intermediate product 10: under ice-bath conditions (eg -20°C to -10°C), N-Boc-AA (amino acid protected by N-terminal tert-butoxycarbonyl group) was dissolved in DMF, and 1 -Hydroxybenzotriazole (HOBt), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI), stir and mix (generally 0.5h to 1h), add The reactant 9a is kept stirring for a period of time (for example, 0.5-2 hours, such as 1 hour) and then turned to room temperature until the reaction is complete (generally, the reaction can be completed in 4-10 hours). Add ethyl acetate to extract and wash with water at the same time, further wash the extracted product with saturated brine, then dry over anhydrous sodium sulfate, concentrate, and if necessary, further purify by column chromatography to obtain the product 10; ...
Embodiment 2
[0096] (S)-N-(2,6-dimethoxypyridin-3-yl)-2-(2-amino-3-(4-hydroxy)phenyl-propionylamino)-N-methyl-6 -Synthesis of carbazolesulfonamide trifluoroacetate (11b)
[0097] The synthetic process is referring to embodiment 1, and specific conditions are as follows:
[0098] Step (1): Synthesis of (S)-N-(2,6-dimethoxypyridin-3-yl)-2-(2-tert-butoxyamido-3-(4-hydroxy)phenyl-propane Amino)-N-methyl-6-carbazolesulfonamide (10a)
[0099]
[0100] Reactant 9a (200mg, 0.485mmol), Boc-L-tyrosine (Boc-L-Try, 164mg, 0.582mmol), HOBt (78.6mg, 0.582mmol), EDCI (139mg, 0.73mmol), dissolved in 10 mL of DMF, reacted for 6 h, and column chromatography (PE:EA=1:1) gave 311 mg of the product (10a).
[0101] Yield: 95%.
[0102] 1 H NMR (500MHz, DMSO-d 6 )δppm 10.26(s,1H),9.32(s,1H),9.19(s,1H),8.41(s,1H),8.17(d,J=8.4Hz,1H),8.07(s,1H),7.72 (s,2H),7.45(d,J=8.3Hz,1H),7.39(d,J=8.1Hz,1H),7.15(d,J=7.7Hz,2H),7.09(d,J=7.8Hz ,1H),6.69(d,J=7.9Hz,2H),6.32(d,J=8.3Hz,1H),4.33(s,1H),3.89(s,3H),3.75(s,3H),3....
Embodiment 3
[0110] Synthesis of N-(2,6-dimethoxypyridin-3-yl)-2-(2-amino-acetylamino)-N-methyl-6-carbazolesulfonamide·hydrochloride (11c)
[0111] The synthetic process is referring to embodiment 1, and specific conditions are as follows:
[0112] Step (1): Synthesis of N-(2,6-dimethoxypyridin-3-yl)-2-(2-tert-butoxyamido-acetylamino)-N-methyl-6-carbazolesulfonamide (10c)
[0113]
[0114] Reactant 9a (200mg, 0.485mmol), Boc-glycine (11mg, 0.582mmol), HOBt (79mg, 0.582mmol), EDCI (139mg, 0.727mmol) were dissolved in 10mLDMF, reacted for 6h, column chromatography (PE:EA =1:3) yielded 260 mg of product (10c).
[0115] Yield: 96.7%.
[0116] 1 H NMR (500MHz, DMSO-d 6 )δppm: 10.17(s, 1H), 9.29(s, 1H), 8.38(s, 1H), 8.14(d, J=8.3Hz, 1H), 8.09(s, 1H), 7.69(s, 2H), 7.41(d,J=8.3Hz,1H),7.33(s,1H),7.08(s,1H),6.29(d,J=7.9Hz,1H),3.86(s,3H),3.77(d,J =25.1Hz, 3H), 3.73(s, 3H), 3.43(s, 3H), 1.41(s, 9H).
[0117] 13 C NMR (101MHz, DMSO-d 6)δppm: 172.26, 143.02–142.82, 139.82–139.62, 127.89, 11...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com