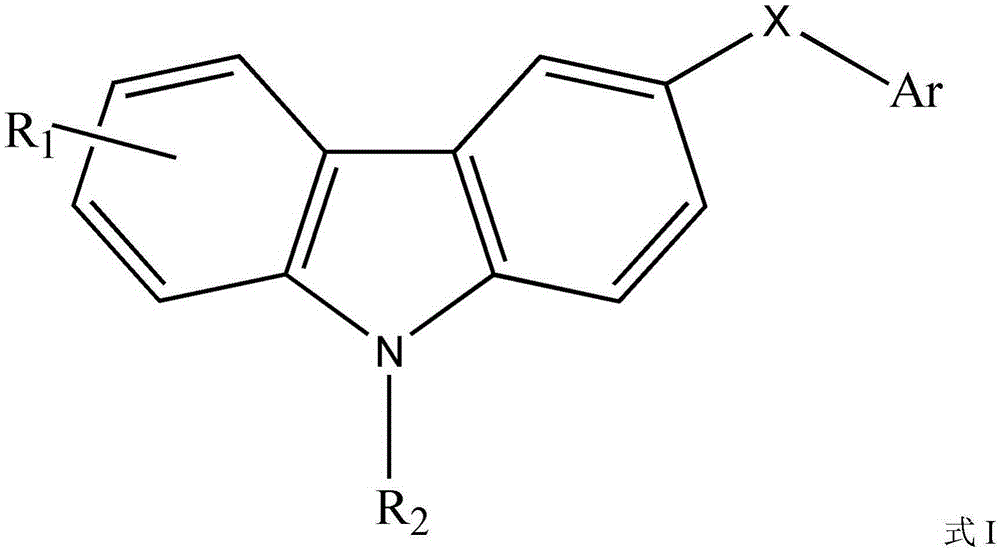
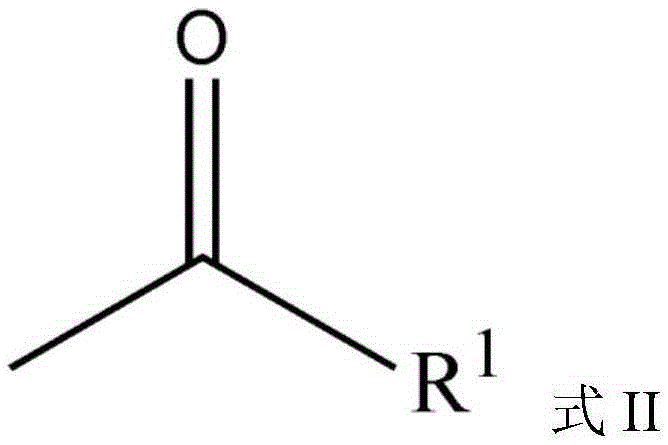
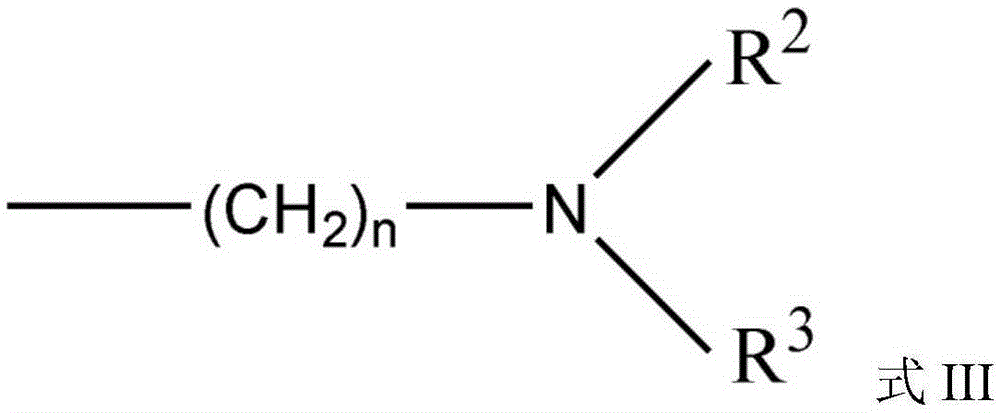
High-dispersion carbazole sulfonamide derivatives and preparation method thereof
A technology of carbazole sulfonamide and derivatives, which is applied in the field of chemistry, can solve the problems of insufficient dissolution rate and insoluble in water, etc., and achieve the effect of improving dissolution rate and solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Example 1 Preparation of nanoparticles loaded with carbazole sulfonamide derivatives
[0084] 1g of the active pharmaceutical ingredient compound 1 was dissolved in 20mL of ethanol, and then 10g of nano zinc oxide A was added. After mixing and stirring, sonicate for 1 min. Rotary evaporation was performed under reduced pressure at 40°C until the solvent evaporated completely. Vacuum dry for 2 hours, pass through 80 mesh sieve after grinding, and the obtained solid particles are recorded as sample 1-1 # .
[0085] Sample 1-2 # ~ Sample 1-21 # The preparation operation and sample 1-1 # The same, the difference is the type of raw material, dosage, evaporation temperature, sample 1-1 # ~ Sample 1-21 # The various substance types, consumption and evaporation temperature of the present invention are shown in Table 3. Among them, sample 1-1 # ~ Sample 1-21 # The amount of inorganic nanocarriers is 10g.
[0086] table 3
[0087]
[0088]
Embodiment 2
[0089] Example 2 Preparation of nanoparticles loaded with carbazole sulfonamide derivatives
[0090] With sample 1-1 in embodiment 1 # The preparation steps are the same, except that, in addition to compound 1 and nano-zinc oxide A, 1.5 g of poloxamer 188 is added to 20 mL of ethanol. After mixing and stirring, ultrasonic treatment was performed for 5 minutes, then rotary evaporation under reduced pressure at 50°C until the solvent evaporated completely, and vacuum drying for 2 hours. The obtained solid was recorded as sample 2-1 # .
[0091] Sample 2-2 # ~Sample 2-21 # The preparation operation was the same as that of sample 2-1 # The same, the difference is the amount of poloxamer 188 added and the evaporation temperature, sample 2-1 # ~Sample 2-21 # The various substance types, consumption and evaporation temperature of the present invention are shown in Table 4. Among them, sample 2-1 # ~Sample 2-21 # The amount of inorganic nanocarriers is 10g.
[0092] Table 4 ...
Embodiment 3
[0095] Embodiment 3 tablet preparation
[0096] Tablet 1-1 # Preparation of:
[0097] 50g sample 1-1 # After grinding and mixing with 150 g of beta-cyclodextrin, 350 g of lactose powder were added. Then add 22 g of croscarmellose sodium and 0.2 g of aspartame through a 100-mesh sieve, and mix well. Add 5% polyvinylpyrrolidone aqueous solution until a soft material can be obtained, then granulate with a 20-mesh sieve, dry at 80°C, and granulate with a 18-mesh sieve. The obtained granules were mixed with 6 g of micropowder silica gel, and pressed into tablets. Made in 1000 pieces.
[0098] Tablet 1-2 # ~ Tablets 1-21 # and tablet 2-1 # ~ Tablets 2-21 # Preparation of:
[0099] Preparation of Tablets 1-2 # ~ Tablets 1-21 # and tablet 2-1 # ~ Tablets 2-21 # The course of operation with the tablet 1-1 # The preparation process is the same, just sample 1-1 # Change to sample 1-2 # ~ Sample 1-21 # and sample 2-1 # ~Sample 2-21 # .
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com