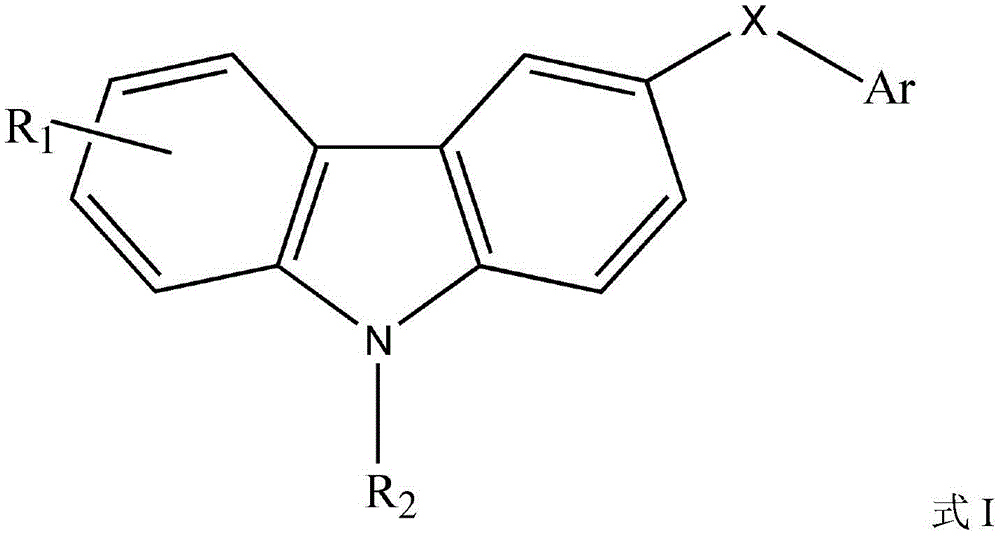
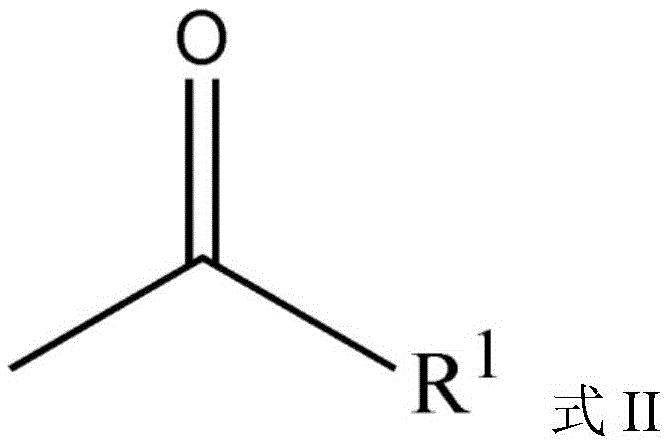
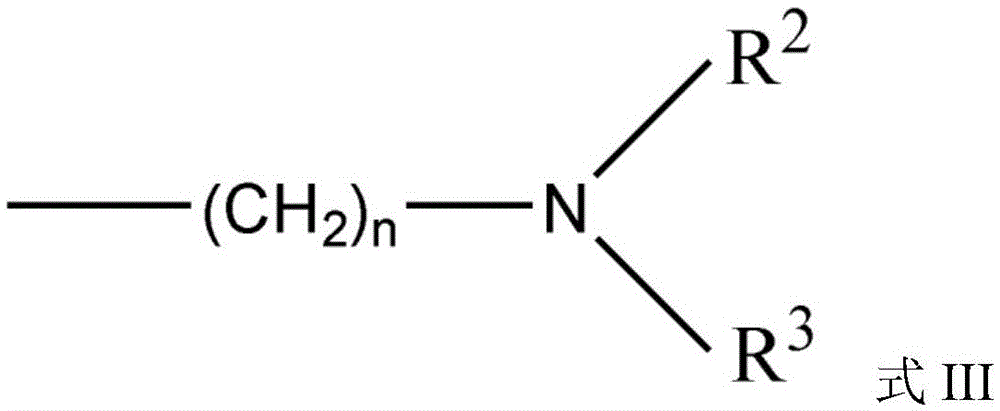
Carbazole sulfonamide derivative eutectic and preparation method thereof
A technology of carbazole sulfonamide and derivatives, applied in the chemical field, can solve problems such as poor solubility in water and insufficient dissolution rate, and achieve the effects of high tolerance, high dissolution rate and solubility, and high dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Embodiment 1 Evaporation solvent method prepares carbazole sulfonamide derivative eutectic
[0073] Add 0.1 mmol of the active pharmaceutical ingredient Compound 1 and 0.1 mmol of the guest molecule glutaric acid into 10 mL of the solvent tetrahydrofuran. After mixing and stirring for 15 minutes, let it stand for 30 minutes, filter, put the filtrate into a 25mL test tube, seal it with plastic wrap, pierce the hole, and place it at 25°C for natural volatilization. After 1 week, a solid-liquid mixture containing massive crystals was obtained. The resulting solid-liquid mixture was filtered to collect massive crystals, and dried at 60°C for 12 hours, and the obtained crystals were designated as sample 1-1 # .
[0074] Sample 1-2 # ~ Sample 1-21 # The preparation operation and sample 1-1 # The same, the difference is the type of raw material, dosage and volatilization temperature, sample 1-1 # ~ Sample 1-21 # See Table 1 for each substance type, consumption and solven...
Embodiment 2
[0078] Example 2 Preparation of Carbazole Sulfonamide Derivatives Eutectic by Mixed Grinding Method
[0079] Add 0.1 mmol active pharmaceutical ingredient compound 1, 0.1 mmol guest molecule glutaric acid, and 0.1 mL ethanol into the mixing ball mill. Ball mill at room temperature for 30 minutes, and then dry at 60°C for 12 hours. The obtained solid sample is designated as sample 2-1 # .
[0080] Sample 2-2 # ~ Sample 2-13 # The preparation operation and sample 2-1 # The same, but the difference is the type of raw material, dosage and ball milling temperature. Sample 2-1 # ~ Sample 2-13 # See Table 2 for the types, amounts and milling temperatures of each material.
[0081] Add 0.1 mmol of compound 10 and 0.1 mmol of glutaric acid into the mixing ball mill. Ball milled at room temperature for 30 minutes, and the obtained solid sample was recorded as sample 2-14 # .
[0082] Sample 2-15 # ~Sample 2-25 # The preparative operation with samples 2-14 # The same, but t...
Embodiment 313
[0086] Example 3 13 C-NMR crystal characterization
[0087] Take oxalic acid crystals, sodium oxalate crystals, malonic acid crystals, sodium malonate crystals, glutaric acid crystals, sodium glutarate crystals, maleic acid crystals, and sodium maleate crystals respectively. 13 C-NMR analysis of -COOH of organic acids and -COO of organic acid salts - The chemical shift, as a benchmark, the results are shown in Table 3.
[0088] table 3
[0089] organic acid
[0090] The sample 1-1 that obtains to embodiment 1 # ~ Sample 1-21 # And the sample 2-1 that embodiment 2 obtains # ~Sample 2-25 # conduct 13 In C-NMR analysis, the peak positions near the chemical shift of carboxyl-COOH are consistent with the organic acids used in the corresponding samples, that is, carboxyl-COOH can be detected in the above samples. Carboxylic acid and -COO were not detected in the above samples - chemical shift peaks. Description Sample 1-1 # ~ Sample 1-21 # Crystals and samples ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com